Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
ORIGINAL PAPER
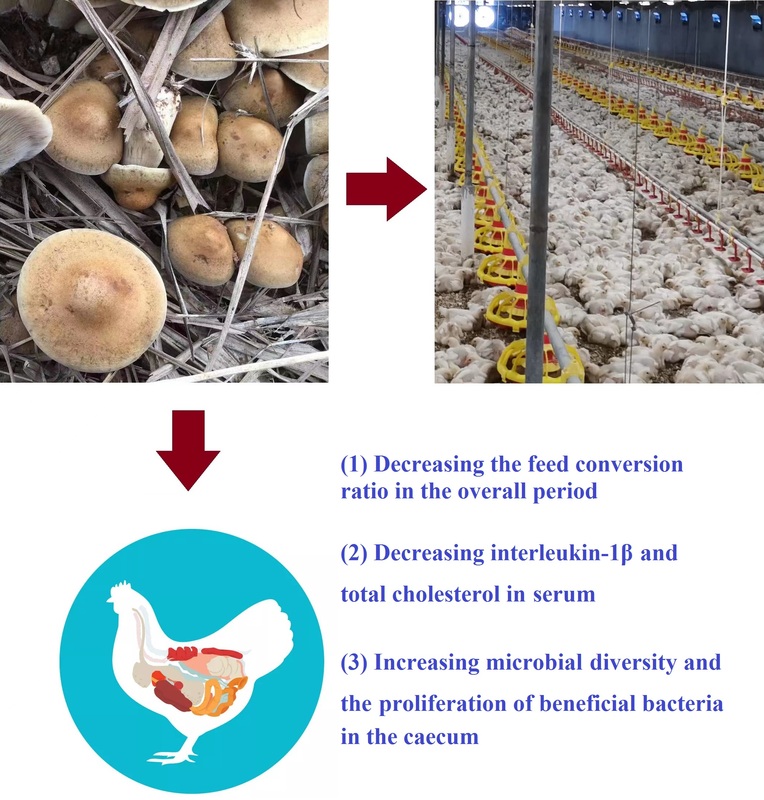
Agaricus blazei Murrill stipe promotes growth by improving anti-inflammatory activity and gut function in broilers
1
Guangxi Academy of Agricultural Sciences, Guangxi Crop Genetic Improvement and Biotechnology Laboratory, 530007, Nanning, China
2
Guangxi Academy of Agricultural Sciences, Institute of Microbiology, 530007, Nanning, China
Publication date: 2023-07-24
J. Anim. Feed Sci. 2024;33(1):64-75
KEYWORDS
TOPICS
ABSTRACT
Agaricus blazei Murrill (AB) is a mushroom known for its medicinal
applications. AB stipe (ABS) is a by-product of the mushroom’s processing,
which is rich in bioactive substances, such as polysaccharides. This experiment
was conducted to assess the effects of dietary ABS supplementation on growth
performance, short-chain fatty acid production, antioxidant capacity, immune
function, meat quality, gut function, and caecal microbiota in broilers. A total of
180 one-day-old male Arbor Acre broilers (46.17 ± 2.47 g) were randomised
to 3 treatments, with 6 replicates per treatment and 10 broilers per replicate.
The treatments included one basal diet (control group) and two experimental
diets supplemented with 1% and 2% ABS, respectively. This trial lasted
42 days and was divided into Phase 1 (day 1–21) and Phase 2 (day 22–42).
The results showed that broilers fed ABS exhibited a significant reduction in
the feed conversion ratio throughout the study period (P < 0.05), and tended to
have increased average daily gain in Phase 2 (P < 0.1) and the overall period
(P < 0.1). On day 42, dietary ABS supplementation significantly decreased
the concentration of interleukin-1β (P < 0.05) and total cholesterol in serum
(P < 0.05). Moreover, ABS addition significantly increased the villus height-tocrypt
depth ratio (P < 0.05) in the jejunum and tended to increase villus height
(P < 0.1) and decrease crypt depth (P < 0.1). In addition, the ABS-supplemented
groups showed higher microbial diversity and increased proliferation of beneficial
bacteria in the caecum. Based on these findings, it can be concluded that ABS
supplementation can improve growth performance, anti-inflammatory activity,
and intestinal function.
FUNDING
This research was financially supported by the Science and Technology Base and Talent Project of Guangxi (Grant No. Guike AA21196003), China Agriculture Research System (CARS20), Special Fund for Basic Scientific Research of the Guangxi Academy of Agricultural Sciences (2023YM92), Science and Technology Development Fund of the Guangxi Academy of Agricultural Sciences (2023YM103).
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
REFERENCES (54)
1.
Abdelqader A., Al-Fataftah A.-R., 2016. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest. Sci. 183, 78–83, https://doi.org/10.1016/j.livs....
2.
Angkanaporn K., Choct M., Bryden W.L., Annison E.F., Annison G., 1994. Effects of wheat pentosans on endogenous amino acid losses in chickens. J. Sci. Food Agric. 66, 399–404, https://doi.org/10.1002/JSFA.2....
3.
AOAC International, 2006. Official methods of analysis of AOAC International. 18th Edition. AOAC Press. Arlington, VA (USA).
4.
Bernad-Roche M., Bellés A., Grasa L., Casanova-Higes A., Mainar-Jaime R.C., 2021. Effects of dietary supplementation with protected sodium butyrate on gut microbiota in growing-finishing pigs. Animals 11, 2137, https://doi.org/10.3390/ani110....
5.
Bian M., Wang J., Wang Y., Nie A., Zhu C., Sun Z., Zhou Z., Zhang B., 2020. Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. Biomed. Pharmacother. 131, 110719, https://doi.org/10.1016/j.biop....
6.
Bonvegna S., Cilia R., 2023. Chapter 8 – Disease mechanisms as subtypes: Microbiome. In: A.J. Espay (Editor). Handbook of Clinical Neurology. Elsevier Press. Amsterdam (Netherlands), pp.107–131, https://doi.org/10.1016/B978-0...
7.
Ciobanu D.C., Bastiaansen J.W., Lonergan S.M., Thomsen H., Dekkers J.C., Plastow G.S., Rothschild M.F., 2004. New alleles in calpastatin gene are associated with meat quality traits in pigs. J. Anim. Sci. 82, 2829–2839, https://doi.org/10.2527/2004.8....
8.
Da Silva A.F., Sartori D., Macedo F.C. Jr., Ribeiro L.R., Fungaro M.H., Mantovani M.S., 2013. Effects of β-glucan extracted from Agaricus blazei on the expression of ERCC5, CASP9, and CYP1A1 genes and metabolic profile in HepG2 cells. Hum. Exp. Toxicol. 32, 647–654, https://doi.org/10.1177/096032....
9.
Dechesne A., Musovic S., Palomo A., Diwan V., Smets B.F., 2016. Underestimation of ammonia-oxidizing bacteria abundance by amplification bias in amoA-targeted qPCR. Microb. Biotechnol. 9, 519–524, https://doi.org/10.1111/1751-7....
10.
Delmanto R.D., de Lima P.L., Sugui M.M., da Eira A.F., Salvadori D.M., Speit G., Ribeiro L.R., 2001. Antimutagenic effect of Agaricus blazei Murrill mushroom on the genotoxicity induced by cyclophosphamide. Mutat. Res. 496, 15–21, https://doi.org/10.1016/s1383-....
11.
Endo M., Beppu H., Akiyama H., Wakamatsu K., Ito S., Kawamoto Y., Shimpo K., Sumiya T., Koike T., Matsui T., 2010. Agaritine purified from Agaricus blazei Murrill exerts anti-tumor activity against leukemic cells. Biochim. Biophys. Acta 1800, 669–673, https://doi.org/10.1016/j.bbag....
12.
Fard S.H., Toghyani M., Tabeidian S.A., 2014. Effect of oyster mushroom wastes on performance, immune responses and intestinal morphology of broiler chickens. Int. J. Recycling Org. Waste Agric. 3, 141–146, https://doi.org/10.1007/s40093....
13.
Giannenas I., Pappas I.S., Mavridis S., Kontopidis G., Skoufos J., Kyriazakis I., 2010a. Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poult. Sci. 89, 303–311, https://doi.org/10.3382/ps.200....
14.
Giannenas I., Tontis D., Tsalie E., Chronis E.F., Doukas D., Kyriazakis I., 2010b. Influence of dietary mushroom Agaricus bisporus on intestinal morphology and microflora composition in broiler chickens. Res. Vet. Sci. 89, 78–84, https://doi.org/10.1016/j.rvsc....
15.
González-Alvarado J.M., Jiménez-Moreno E., Lázaro R., Mateos G.G., 2007. Effect of type of cereal, heat processing of the cereal, and inclusion of fiber in the diet on productive performance and digestive traits of broilers. Poult. Sci. 86, 1705–1715, https://doi.org/10.1093/ps/86.....
16.
Guo F.C., Kwakkel R.P., Williams B.A., Li W.K., Li H.S., Luo J.Y., Li X.P., Wei Y.X., Yan Z.T., Verstegen M.W., 2004. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on growth performance of broilers. Br. Poult. Sci. 45, 684–694, https://doi.org/10.1080/000716....
17.
Guo F.C., Savelkoul H.F.J., Kwakkel R.P., Williams B.A., Verstegen M.W.A., 2003. Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World’s Poult. Sci. J. 59, 427–440, https://doi.org/10.1079/WPS200....
18.
Hasegawa Y., Kawai M., Bessho K. et al., 2019. CYP7A1 expression in hepatocytes is retained with upregulated fibroblast growth factor 19 in pediatric biliary atresia. Hepatol. Res. 49, 314–323, https://doi.org/10.1111/hepr.1....
19.
Hu Z.Q., Yao Y.X., Lv M., Zhang Y.Q., Zhang L., Yuan Y.H., Yue T.L., 2021. Isolation and identification of three water-soluble selenoproteins in Se-enriched Agaricus blazei Murrill. Food Chem. 344, 128691, https://doi.org/10.1016/j.food....
20.
Jia S.Y., Li F., Liu Y., Ren H.T., Gong G.L., Wang Y.Y., Wu S.H., 2013. Effects of extraction methods on the antioxidant activities of polysaccharides from Agaricus blazei Murrill. Int. J. Biol. Macromol. 62, 66–69, https://doi.org/10.1016/j.ijbi....
21.
Johnson E., Førland D.T., Saetre L., Bernardshaw S.V., Lyberg T., Hetland G., 2009. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immunol. 69, 242–250, https://doi.org/10.1111/j.1365....
22.
Kaneno R., Fontanari L.M., Santos S.A., Di Stasi L.C., Rodrigues Filho E., Eira A.F., 2004. Effects of extracts from Brazilian sun-mushroom (Agaricus blazei) on the NK activity and lymphoproliferative responsiveness of Ehrlich tumor-bearing mice. Food Chem. Toxicol. 42, 909–916, https://doi.org/10.1016/j.fct.....
23.
Kuroiwa Y., Nishikawa A., Imazawa T., Kanki K., Kitamura Y., Umemura T., Hirose M., 2005. Lack of subchronic toxicity of an aqueous extract of Agaricus blazei Murrill in F344 rats. Food Chem. Toxicol. 43, 1047–1053, https://doi.org/10.1016/j.fct.....
24.
Lee EA., Lee DI., Kim H.Y., Ahn S.H., Seong H.R., Jung W.H., Kim K.Y., Kim S., Rhee S.D., 2018. Cyp7a1 is continuously increased with disrupted Fxr-mediated feedback inhibition in hypercholesterolemic TALLYHO/Jng mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 20–25, https://doi.org/10.1016/j.bbal....
25.
Lee M.T., Lin W.C., Lee T.T., 2019. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals – a review. Asian Australas. J. Anim. Sci. 32, 309–319, https://doi.org/10.5713/ajas.1....
26.
Lima C.U.J.O., Gris E.F., Karnikowski M.G.O., 2016. Antimicrobial properties of the mushroom Agaricus blazei – integrative review. Rev. Bras. Farmacogn. 26, 780–786, https://doi.org/10.1016/j.bjp.....
27.
Li Y.X., Lu X.C., Li X., Guo X., Shen Y., Li Y.N., Xu G.Y., Han X., An L.P., Du P.G., 2020a. Effects of Agaricus blazei Murrill polysaccharides on hyperlipidemic rats by regulation of intestinal microflora. Food Sci. Nutr. 8, 2758–2772, https://doi.org/10.1002/fsn3.1....
28.
Li Y.X., Sheng Y., Lu X.C., Guo X., Xu G.Y., Han X., An L.P., Du P.G., 2020b. Isolation and purification of acidic polysaccharides from Agaricus blazei Murill and evaluation of their lipid-lowering mechanism. Int. J. Biol. Macromol. 157, 276–287, https://doi.org/10.1016/j.ijbi....
29.
Lin Y., Olukosi O.A., 2021. Qualitative and quantitative profiles of jejunal oligosaccharides and cecal short-chain fatty acids in broiler chickens receiving different dietary levels of fiber, protein and exogenous enzymes. J. Sci. Food Agric. 101, 5190–5201, https://doi.org/10.1002/jsfa.1....
30.
Liu X.Z., Zhao J.B., Zhang G., Hu J.X., Liu L., Piao X.S., Zhang S., Li Y., 2020. Dietary supplementation with Flammulina velutipes stem waste on growth performance, fecal short chain fatty acids and serum profile in weaned piglets. Animals 10, 82, https://doi.org/10.3390/ani100....
31.
Maribo H., 2004. Agaricus and Rød Solhat for weaners. National Committee of Pig Product (in Danish). online publication: https://svineproduktion.dk/Pub....
32.
Miles R.D., Butcher G.B., Henry P.R., Littell R.C., 2006. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 85, 476–485, https://doi.org/10.1093/ps/85.....
33.
Montagne L., Pluske J., Hampson D., 2003. A review of interactions between dietary fiber and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 108, 95–117, https://doi.org/10.1016/S0377-....
34.
Moon S.H., Lee I., Feng X., Lee H.Y., Kim J., Ahn D.U., 2016. Effect of dietary beta-glucan on the performance of broilers and the quality of broiler breast meat. Asian-Australas. J. Anim. Sci. 29, 384–389, https://doi.org/10.5713/ajas.1....
35.
NRC (National Research Council), 1994. Nutrient Requirements of Poultry: Ninth Revised Edition. The National Academies Press. Washington DC (USA).
36.
Nielsen S.S. (Editor), 2003. Phenol-sulfuric acid method for total carbohydrates. Food Analysis Laboratory Manual. Food Science Texts Series Chapter 6. Springer Press. Boston, MA (USA), pp.39–44, https://doi.org/10.1007/978-1-...
37.
Ohno N., Furukawa M., Miura N.N., Adachi Y., Motoi M., Yadomae T., 2001. Antitumor β-glucan from the cultured fruit body of Agaricus blazei. Biol. Pharm. Bull. 24, 820–828, https://doi.org/10.1248/bpb.24....
38.
Pluske J.R., Williams I.H., Aherne F.X., 1996. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 62, 131–144, https://doi.org/10.1017/S13577....
39.
Porter M., Murray R., 2001. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 56, 405–411, https://doi.org/10.1046/j.1365....
40.
Shen H.S., Chen J.C., Wu L., Li Y.B., Zhou T., 2012. Biodegrading wheat bran with Agaricus blazei and its effects on intestinal development identified with Mice. J. Integr. Agric. 11, 456–464, https://doi.org/10.1016/S2095-....
41.
Simpson H.L., Campbell B.J., 2015. Review article: dietary fiber-microbiota interactions. Aliment. Pharmacol. Ther. 42, 158–179, https://doi.org/10.1111/apt.13....
42.
Straadt I.K., Rasmussen M., Andersen H.J., Bertram H.C., 2007. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss-A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 75, 687–695, https://doi.org/10.1016/j.meat....
43.
Tzianabos A.O., 2000. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin. Microbiol. Rev. 13, 523–533, https://doi.org/10.1128/CMR.13....
44.
Van Soest P.J., Robertson J.B., Lewis B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597, https://doi.org/10.3168/jds.S0....
45.
Wang J.T., Wang Q., Han J.R., 2013. Yield, polysaccharides content and antioxidant properties of the mushroom Agaricus subrufecens produced on different substrates based on selected agricultural wastes. Sci. Hortic. 157, 84–89, https://doi.org/10.1016/j.scie....
46.
Walugembe M., Hsieh J.C., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F., 2015. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 94, 2351–2359, https://doi.org/10.3382/ps/pev....
47.
Wei Q., Zhan Y., Chen B., Xie B., Fang T., Ravishankar S., Jiang Y., 2019. Assessment of antioxidant and antidiabetic properties of Agaricus blazei Murill extracts. Food Sci. Nutr. 8, 332–339, https://doi.org/10.1002/fsn3.1....
48.
Wu M., Yang S., Wang S., Cao Y., Zhao R., Li X., Xing Y., Liu L., 2020. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front. Pharmacol. 11, 223, https://doi.org/10.3389/fphar.....
49.
Wu S.H., Li F., Jia S.Y., Ren H.T., Gong G.L., Wang Y.Y., Lv Z.S., Liu Y., 2014. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr. Polym. 103, 414–417, https://doi.org/10.1016/j.carb....
50.
Yang Y., Iji P.A., Choct M., 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poult. Sci. J. 65, 97–114, https://doi.org/10.1017/S00439....
51.
Zhai F.H., Wang Q., Han J.R., 2015. Nutritional components and antioxidant properties of seven kinds of cereals fermented by the basidiomycete Agaricus blazei. J. Cereal Sci. 65, 202–208, https://doi.org/10.1016/j.jcs.....
52.
Zhang L., Wang Z., Zhang X. et al., 2022. Alterations of the gut microbiota in patients with diabetic nephropathy. Microbiol. Spectr. 10, e0032422, https://doi.org/10.1128/spectr....
53.
Zhang X., Liu L., Luo J., Peng X., 2022. Anti-aging potency correlates with metabolites from in vitro fermentation of edible fungal polysaccharides using human fecal intestinal microflora. Food Funct. 13, 11592–11603, https://doi.org/10.1039/d2fo01....
54.
Zou X.Y., Zhang M., Tu W.J., Zhang Q., Jin M.L., Fang R.D., Jiang S., 2022. Bacillus subtilis inhibits intestinal inflammation and oxidative stress by regulating gut flora and related metabolites in laying hens. Animal 16, 100474, https://doi.org/10.1016/j.anim....
CITATIONS (1):
1.
Hedgehog Signalling Pathway and Its Role in Shaping the Architecture of Intestinal Epithelium
Adrianna Konopka, Kamil Gawin, Marcin Barszcz
International Journal of Molecular Sciences
Adrianna Konopka, Kamil Gawin, Marcin Barszcz
International Journal of Molecular Sciences
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.