Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
ORIGINAL PAPER
Changes in nuclear factor kappa B components expression in the ovine spleen during early pregnancy
1
Hebei University of Engineering, School of Life Sciences and Food Engineering, No. 19 Taiji Road, Handan 056038, China
Publication date: 2022-02-27
J. Anim. Feed Sci. 2022;31(1):3-11
KEYWORDS
TOPICS
ABSTRACT
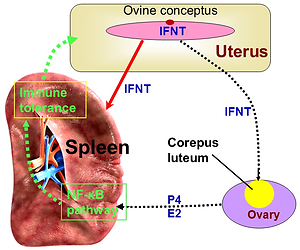
Normal pregnancy is characterised by a systemic immunological
tolerance against foetal antigens, and the spleen contributes to the adaptive
immune tolerance during pregnancy. Nuclear factor kappa B (NF-κB)
signallings participate in splenic immune regulation, but it is unclear whether
there are changes in NF-κB components expression in the ovine spleen
during early pregnancy. The objective of this study was to explore the effects
of early pregnancy on the expression of NF-κB components in the maternal
spleen in sheep. The spleens were sampled on day 16 of the oestrous
cycle, and on days 13, 16 and 25 of gestation. The expression of NF-κB
components, including NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB and
c-Rel, were detected by quantitative real-time PCR, Western blot analysis and
immunohistochemical analysis. The results showed that NF-κB1 and RelB
mRNA and proteins decreased at days 13 and 16 of pregnancy, but increased
at day 25 of pregnancy in comparison with that on day 16 of the oestrous
cycle. Nevertheless, NF-κB2 and RelA mRNA and proteins peaked at days
13 and 16 of pregnancy. In addition, early pregnancy inhibited C-Rel expression
at days 16 to 25 of pregnancy in the maternal spleen. In conclusion, the variable
expression of individual NF-κB components was found in the ovine spleen
during early pregnancy, which may be related with pregnancy recognition, and
essential for the embryo implantation and pregnancy maintenance.
ACKNOWLEDGEMENTS
The current study was supported by the grants
from Natural Science Foundation of Hebei Province,
China (C2021402019), and Hebei Science and
Technology Bureau, China (21326601D).
CONFLICT OF INTEREST
The authors declare that there is no conflict of
interest.
METADATA IN OTHER LANGUAGES:
Chinese
早期妊娠对绵羊脾脏表达核因子-κB组分的影响
摘要
核因子-κB组分, 妊娠, 绵羊, 脾脏
正常妊娠情况下,母体对胎儿抗原产生系统性免疫耐受。在妊娠期间,脾脏参与母体适应性免疫耐受
的形成。核因子-κB (Nuclear factor kappa B, NF-κB) 信号参与脾脏的免疫调节,但是,妊娠早期绵羊脾脏表达
NF-κB组分是否发生改变,目前还不清楚。本研究的目的是探讨早期妊娠对绵羊母体脾脏表达NF-κB组分的
影响。采集发情周期第16天,以及妊娠第13、16和25天的母羊脾脏,采用实时定量PCR、蛋白印迹和免疫
组化分析NF-κB组分的表达,NF-κB组分包括NF-κB1 (p50)、NF-κB2 (p52)、RelA (p65)、RelB和C-Rel。试验结
果表明,与发情周期第16天相比,在妊娠第13天和16天,脾脏表达NF-κB1和 RelB的mRNA和蛋白质减少,
但是,在妊娠第25天却增加。此外,在妊娠第13天和16天,NF-κB2和RelA的mRNA和蛋白质水平达到峰值。
同时,从妊娠第16至25天,母体脾脏C-Rel的表达水平逐步降低。综上所述,在绵羊妊娠早期,脾脏表达
NF-κB不同组分发生改变,这可能与妊娠识别有关,对胚胎附植和妊娠维持至关重要。
REFERENCES (41)
1.
Bai J., Zhang L., Zhao Z., Li N., Wang B., Yang L., 2020. Expression of melatonin receptors and CD4 in the ovine thymus, lymph node, spleen and liver during early pregnancy. Immunology 160, 52–63, https://doi.org/10.1111/imm.13....
2.
Basavarajappa S.C., Ramakrishnan P., 2020. Regulation of B-cell function by NF-kappaB c-Rel in health and disease. Cell Mol. Life Sci. 77, 3325–3340, https://doi.org/10.1007/s00018....
3.
Baud V., Collares D., 2016. Post-translational modifications of RelB NF-κB subunit and associated functions. Cells 5, 22, https://doi.org/10.3390/cells5....
4.
Beinke S., Ley S.C., 2004. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 382, 393–409, https://doi.org/10.1042/BJ2004....
5.
Bozkurt M., Şahin L., Ulaş M., 2015. Hysteroscopic polypectomy decreases NF-κB1 expression in the mid-secretory endometrium of women with endometrial polyp. Eur. J. Obstet. Gynecol. Reprod. Biol. 189, 96–100, https://doi.org/10.1016/j.ejog....
6.
Buska-Mach K., Kedzierska A.E., Lepczynski A. et al., 2021. Differential signals from TNFα-treated and untreated embryos in uterine tissues and splenic CD4+ T lymphocytes during preimplantation pregnancy in mice. Front. Vet. Sci. 8, 641553, https://doi.org/10.3389/fvets.....
7.
Caamaño J.H., Rizzo C.A., Durham S.K., Barton D.S., Raventós-Suárez C., Snapper C.M., Bravo R., 1998. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J. Exp. Med. 187, 185–196, https://doi.org/10.1084/jem.18....
8.
Cao N., Cao L., Gao M., Wang H., Zhang L., Yang L., 2021. Changes in mRNA and protein levels of gonadotropin releasing hormone and receptor in ovine thymus, lymph node, spleen, and liver during early pregnancy. Domest. Anim. Endocrinol. 76, 106607, https://doi.org/10.1016/j.doma....
9.
Chapman N.R., Europe-Finner G.N., Robson S.C., 2004. Expression and deoxyribonucleic acid-binding activity of the nuclear factor kappaB family in the human myometrium during pregnancy and labor. J. Clin. Endocrinol. Metab. 89, 5683–5693, https://doi.org/10.1210/jc.200....
10.
Dai R., Phillips R.A., Ahmed S.A., 2007. Despite inhibition of nuclear localization of NF-kappa B p65, c-Rel, and RelB, 17-beta estradiol up-regulates NF-kappa B signaling in mouse splenocytes: the potential role of Bcl-3. J. Immunol. 179, 1776–1783, https://doi.org/10.4049/jimmun....
11.
Fuhler G.M., 2020. The immune system and microbiome in pregnancy. Best Pract. Res. Clin. Gastroenterol. 44–45, 101671, https://doi.org/10.1016/j.bpg.....
12.
Godkin J.D., Bazer F.W., Moffatt J., Sessions F., Roberts R.M., 1982. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J. Reprod. Fertil. 65, 141–150, https://doi.org/10.1530/jrf.0.....
13.
Hamon M.H., Heap R.B., 1990. Progesterone and oestrogen concentrations in plasma of Barbary sheep (aoudad, Ammotragus lervia) compared with those of domestic sheep and goats during pregnancy. J. Reprod. Fertil. 90, 207–211, https://doi.org/10.1530/jrf.0.....
14.
Heilmann R.M., Allenspach K., 2017. Pattern-recognition receptors: signaling pathways and dysregulation in canine chronic enteropathies-brief review. J. Vet. Diagn. Invest. 29, 781–787, https://doi.org/10.1177/104063....
15.
Kandil D., Leiman G., Allegretta M., Trotman W., Pantanowitz L., Goulart R., Evans M., 2007. Glypican-3 immunocytochemistry in liver fine-needle aspirates: a novel stain to assist in the differentiation of benign and malignant liver lesions. Cancer 111, 316–322, https://doi.org/10.1002/cncr.2....
16.
Kashimura M., 2020. The human spleen as the center of the blood defense system. Int. J. Hematol. 112, 147–158, https://doi.org/10.1007/s12185....
17.
Lee J., Banu S.K., McCracken J.A., Arosh J.A., 2016. Early pregnancy modulates survival and apoptosis pathways in the corpus luteum in sheep. Reproduction 151, 187–202, https://doi.org/10.1530/REP-15....
18.
Li N., Zhao Z., Bai J., Liu B., Mi H., Zhang L., Li G., Yang L., 2019. Characterization of the Th cytokines profile in ovine spleen during early pregnancy. J. Appl. Anim. Res. 47, 386–393,https://doi.org/10.1080/097121....
19.
Livak K.J., Schmittgen T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2....
20.
Masat E., Gasparini C., Agostinis C., Bossi F., Radillo O., De Seta F., Tamassia N., Cassatella M.A., Bulla R., 2015. RelB activation in anti-inflammatory decidual endothelial cells: a master plan to avoid pregnancy failure? Sci. Rep. 5, 14847, https://doi.org/10.1038/srep14....
21.
McNatty K.P., Revefeim K.J., Young A., 1973. Peripheral plasma progesterone concentrations in sheep during the oestrous cycle. J. Endocrinol. 58, 219–225, https://doi.org/10.1677/joe.0.....
22.
Nakada D., Oguro H., Levi B.P., Ryan N., Kitano A., Saitoh Y., Takeichi M., Wendt G.R., Morrison S.J., 2014. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505, 555–558, https://doi.org/10.1038/nature....
23.
Nakamura H., Kimura T., Ogita K., Nakamura T., Takemura M., Shimoya K., Koyama S., Tsujie T., Koyama M., Murata Y.,Y., 2004. NF-kappaB activation at implantation window of the mouse uterus. Am. J. Reprod. Immunol. 51, 16–21, https://doi.org/10.1046/j.8755....
24.
Norton M.T., Fortner K.A., Bizargity P., Bonney E.A., 2009. Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol. Reprod. 81, 457–464, https://doi.org/10.1095/biolre....
25.
Oh S.Y., Hwang J.R., Choi M., Kim Y.M., Kim J.S., Suh Y.L., Choi S.J., Roh C.R., 2020. Autophagy regulates trophoblast invasion by targeting NF-κB activity. Sci. Rep. 10, 14033, https://doi.org/10.1038/s41598....
26.
Olmos-Ortiz A., Déciga-García M., Preciado-Martínez E. et al., 2019. Prolactin decreases LPS-induced inflammatory cytokines by inhibiting TLR-4/NFκB signaling in the human placenta. Mol. Hum. Reprod. 25, 660–667, https://doi.org/10.1093/molehr....
27.
Ott T.L., 2020. Immunological detection of pregnancy: Evidence for systemic immune modulation during early pregnancy in ruminants. Theriogenology 150, 498–503, https://doi.org/10.1016/j.ther....
28.
Patel H., Zaghloul N., Lin K., Liu S.F., Miller E.J., Ahmed M., 2017. Hypoxia-induced activation of specific members of the NF-kB family and its relevance to pulmonary vascular remodeling. Int.J. Biochem. Cell Biol. 92, 141–147, https://doi.org/10.1016/j.bioc....
29.
Prusty B.K., Hedau S., Singh A., Kar P., Das B.C., 2007. Selective suppression of NF-kBp65 in hepatitis virus-infected pregnant women manifesting severe liver damage and high mortality. Mol. Med. 13, 518–526, https://doi.org/10.2119/2007-0....
30.
Rocha C.C., da Silveira J.C., Forde N., Binelli M., Pugliesi G., 2021. Conceptus-modulated innate immune function during early pregnancy in ruminants: a review. Anim. Reprod. 18, e20200048, https://doi.org/10.1590/1984-3....
31.
Ross J.W., Ashworth M.D., Mathew D., Reagan P., Ritchey J.W., Hayashi K., Spencer T.E., Lucy M., Geisert R.D., 2010. Activation of the transcription factor, nuclear factor kappa-B, during the estrous cycle and early pregnancy in the pig. Reprod. Biol.Endocrinol. 8, 39, https://doi.org/10.1186/1477-7....
32.
Saha I., Jaiswal H., Mishra R. et al., 2020. RelB suppresses type I Interferon signaling in dendritic cells. Cell Immunol. 349, 104043, https://doi.org/10.1016/j.cell....
33.
Sekiya Y., Yamamoto E., Niimi K., Nishino K., Nakamura K., Kotani T., Kajiyama H., Shibata K., Kikkawa F., 2017. c-Rel promotes invasion of choriocarcinoma cells via PI3K/AKT signaling. Oncology 92, 299–310, https://doi.org/10.1159/000458....
34.
Shukla V., Kaushal J.B., Sankhwar P., Manohar M., Dwivedi A., 2019. Inhibition of TPPP3 attenuates β-catenin/NF-κB/COX-2 signaling in endometrial stromal cells and impairs decidualization. J. Endocrinol. 240, 417–429, https://doi.org/10.1530/JOE-18....
35.
Spencer T.E., Forde N., Lonergan P., 2016. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J. Dairy Sci. 99, 5941–5950, https://doi.org/10.3168/jds.20....
36.
Wang Y., Han X., Zhang L., Cao N., Cao L., Yang L., 2019. Early pregnancy induces expression of STAT1, OAS1 and CXCL10 in ovine spleen. Animals 9, 882, https://doi.org/10.3390/ani911....
37.
Wirasinha R.C., Davies A.R., Srivastava M. et al., 2021. Nfkb2 variants reveal a p100-degradation threshold that defines autoimmune susceptibility. J. Exp. Med. 218, e20200476, https://doi.org/10.1084/jem.20....
38.
Xue P., Fan W., Diao Z. et al., 2020. Up-regulation of PTEN via LPS/AP-1/NF-κB pathway inhibits trophoblast invasion contributing to preeclampsia. Mol. Immunol. 118, 182–190, https://doi.org/10.1016/j.moli....
39.
Yamazaki T., Kurosaki T., 2003. Contribution of BCAP to maintenance of mature B cells through c-Rel. Nat. Immunol. 4, 780– 86,https://doi.org/10.1038/ni949.
40.
Yang L., Guo R., Yao X., Yan J., Bai Y., Zhang L., 2018. Expression of progesterone receptor and progesterone-induced blocking factor in the spleen during early pregnancy in ewes. Livest. Sci. 209, 14–19, https://doi.org/10.1016/j.livs....
41.
Yang L., Liu Y., Lv W., Wang P., Wang B., Xue J., Zhang L., 2018. Expression of interferon-stimulated gene 15-kDa protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim. Sci. J. 89, 1540–1548, https://doi.org/10.1111/asj.13....
CITATIONS (7):
1.
Expression of nuclear factor kappa B in ovine maternal inguinal lymph nodes during early pregnancy
Leying Zhang, Taipeng Zhang, Zhen Yang, Chunjiang Cai, Shaopeng Hao, Ling Yang
BMC Veterinary Research
Leying Zhang, Taipeng Zhang, Zhen Yang, Chunjiang Cai, Shaopeng Hao, Ling Yang
BMC Veterinary Research
2.
Early Pregnancy Regulates Expression of IkappaB Family in Ovine Spleen and Lymph Nodes
Shengya Fang, Chunjiang Cai, Ying Bai, Leying Zhang, Ling Yang
International Journal of Molecular Sciences
Shengya Fang, Chunjiang Cai, Ying Bai, Leying Zhang, Ling Yang
International Journal of Molecular Sciences
3.
Expression of IkappaB Family in the Ovine Liver during Early Pregnancy
Chunjiang Cai, Ying Ren, Jianhua Cao, Shengya Fang, Leying Zhang, Ling Yang
Animals
Chunjiang Cai, Ying Ren, Jianhua Cao, Shengya Fang, Leying Zhang, Ling Yang
Animals
4.
Changes in expression levels of Nod-like receptors in the spleen of ewes
Jiaxuan Wu, Shengya Fang, Pengfei Feng, Chunjiang Cai, Leying Zhang, Ling Yang
Animal Reproduction
Jiaxuan Wu, Shengya Fang, Pengfei Feng, Chunjiang Cai, Leying Zhang, Ling Yang
Animal Reproduction
5.
Organic and Inorganic Selenium Compounds Affected Lipidomic Profile of Spleen of Lambs Fed with Diets Enriched in Carnosic Acid and Fish Oil
Małgorzata Białek, Agnieszka Białek, Wiktoria Wojtak, Marian Czauderna
Animals
Małgorzata Białek, Agnieszka Białek, Wiktoria Wojtak, Marian Czauderna
Animals
6.
Exploring the Influence of the Selected Conjugated Fatty Acids Isomers and Cancerous Process on the Fatty Acids Profile of Spleen
Tomasz Lepionka, Małgorzata Białek, Marian Czauderna, Wiktoria Wojtak, Ewelina Maculewicz, Agnieszka Białek
Cancers
Tomasz Lepionka, Małgorzata Białek, Marian Czauderna, Wiktoria Wojtak, Ewelina Maculewicz, Agnieszka Białek
Cancers
7.
Splenic Elemental Composition of Breast Cancer-Suffering Rats Supplemented with Pomegranate Seed Oil and Bitter Melon Extract
Małgorzata Białek, Tomasz Lepionka, Wiktoria Wojtak, Anna Ruszczyńska, Ewa Bulska, Marian Czauderna, Agnieszka Białek
Molecules
Małgorzata Białek, Tomasz Lepionka, Wiktoria Wojtak, Anna Ruszczyńska, Ewa Bulska, Marian Czauderna, Agnieszka Białek
Molecules
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.