Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
ORIGINAL PAPER
Comparing the chemical composition of lesser duckweed
(Lemna minor L.) grown in natural and laboratory settings
1
The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, 05-110 Jabłonna, Poland
Publication date: 2024-06-18
Corresponding author
B. Kowalik
The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, 05-110 Jabłonna, Poland
The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, 05-110 Jabłonna, Poland
J. Anim. Feed Sci. 2024;33(3):357-367
KEYWORDS
TOPICS
ABSTRACT
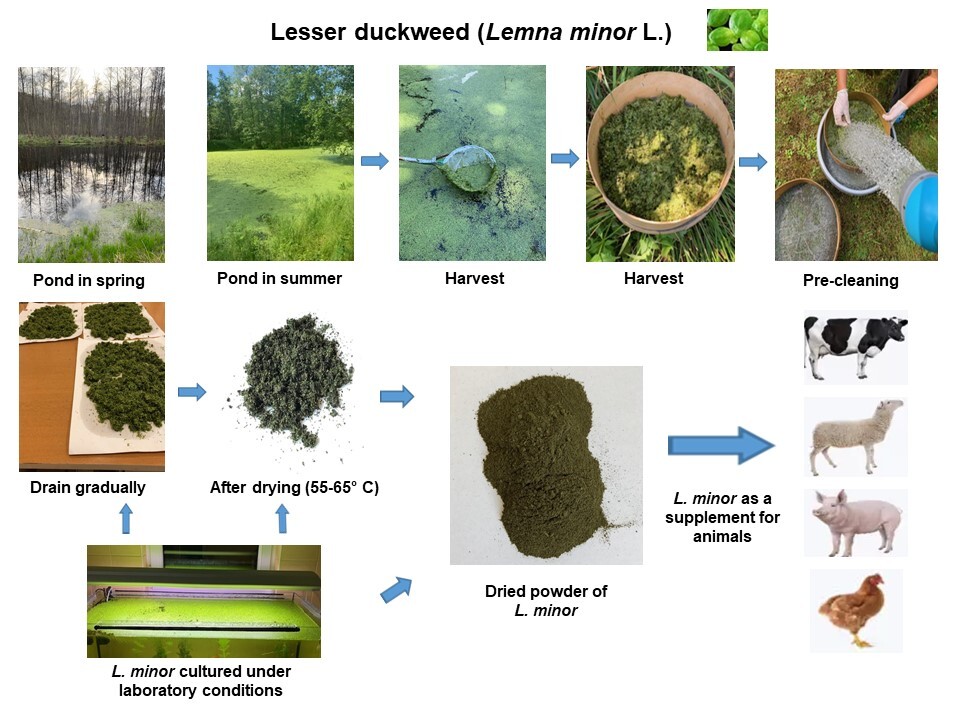
The aim of this study was to compare the chemical composition of lesser duckweed (Lemna minor L.) obtained from the natural environment and cultured under laboratory conditions. In the fi rst variant, lesser duckweed was collected from a natural pond (LDN). Subsequently, the plants were cleaned of other aquatic flora and fauna, dried, and ground. In the second variant, lesser duckweed was cultured in an aquarium (LDC) illuminated from 04:00 to 24:00. The water temperature was 25 °C and the pH was maintained in the range of
6.5–7.3. The content of crude protein, fat, and fibre was similar in LDN and LDC, but the proportion of crude ash was higher in LDC. The content of total amino acids was 95.70 and 68.71 g/100 g crude protein in LDC and LDN, respectively. The concentrations of essential amino acids and nonessential amino acids were 43.75 and 51.96 g/100 g crude protein in LDC, respectively, and 32.10 and 36.61 g/100 g crude protein in LDN, respectively. Palmitic and stearic acids were found in higher quantities in LDN than LDC, whereas the oleic acid content was three-fold higher in LDN compared to LDC. Moreover, linoleic and α-linolenic acid concentrations were higher in LDN than LDC. Mineral analysis revealed elevated levels of Ca, Na, and Zn, while P and Mg levels were lower in LDC. Additionally, the levels of δ-, γ-tocopherols and α-tocotrienol were found to be higher in LDC, while δ- and γ-tocotrienols in LDC were below detectable limits. So, the significant influence of growth conditions on the nutrient composition of L. minor was shown. Optimizing growth conditions is pivotal for enhancing lesser duckweed production. It seems that L. minor can be a valuable source of essential nutrients and could serve as a supplementary food source for both domestic animals and humans. Nonetheless, anti-nutritional components, including toxic metals, should be monitored.
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
REFERENCES (55)
1.
Aguilera-Morales M., Canales-Martinez M.M., Avila-Gonzalez E., Flores-Ortiz X.M., 2018. Nutrients and bioactive compounds of the Lemna gibba and Ulva lactuca as possible ingredients to functional foods. Lat. Am. J. Aquatic Res. 46, 709–716, http://dx.doi.org/10.3856/vol4...
2.
AOAC International, 2011. Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International. 18th ed. Arlington, VA (USA).
3.
Appenroth K.-J., Sowjanya Sree K., Böhmc V., Hammann S., Vetterd W., Leiterere M., Jahreis G., 2017. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 217, 266–273, https://doi.org/10.1016/j.food....
4.
Appenroth K.-J., Sree K.S., Bog M., Ecker J., et al., 2018. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human. Food. Front. Chem. 6, 483, https://doi.org/10.3389/fchem.....
5.
Bhanthumnavin K., Mcgarry M.G., 1971. Wolffia arrhiza as a possible source of inexpensive protein. Nature. 232, 495, https://doi.org/10.1038/232495....
6.
Białek M., Czauderna M., Białek A., 2018. Partial replacement of rapeseed oil with fish oil, and dietary antioxidants supplementation affects concentrations of biohydrogenation products and conjugated fatty acids in rumen and selected lamb tissues. Anim. Feed Sci. Technol. 241, 63–74, https://doi.org/10.1016/j.anif....
7.
Boccardo A., Compiani R, Baldi G., Pravettoni D., Grossi S., Sala G., Taylor S., Neville E., Sgoifo C.A., 2022. Rossi effects of a supplemental calcareous marine algae bolus on blood calcium concentration in dairy heifers. J. Anim. Feed Sci. 31, 1, 40–45, https://doi.org/10.22358/jafs/....
8.
Ceschin S., Leacche I., Pascucci S., Abati S., 2016. Morphological study of Lemna minuta Kunth, an alien species often mistaken for the native L. minor L. (Araceae). Aquat. Bot. 131, 51–56, https://doi.org/10.1016/j.aqua....
9.
Chen Q., Jin Y., Zhang G., Fang Y., Xiao Y., Zhao H., 2012. Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energy 5, 3019–3032, https://doi.org/10.3390/en5083....
10.
Czauderna M., Kowalczyk J., 2007. Alkaline saponification results in decomposition of tocopherols in milk and ovine blood plasma. J. Chromatogr. B, 858, 8–12, https://doi.org/10.1016/j.jchr....
11.
Czauderna M., Wojtak W., Białek M., Białek A., 2024. Optimization of high-efficient pre-column sample treatments and C18-UFLC method for selective quantification of selected chemical forms of tocopherol and tocotrienol in diverse foods. Food Chem. 437, 2, 137909, https://doi.org/10.1016/j.food....
12.
Czerpak R., Piotrowska A., 2005. Wolffia arrhiza – the smallest plant with the highest adaptation ability and applications (in Polish). Kosmos. Probl. Nauk Biol., 54, 241–250.
13.
Dewanji A., 1993. Amino acid composition of leaf proteins extracted from some aquatic weeds. J. Agric. Food Chem. 41, 1232–1236, https://doi.org/10.1021/jf0003....
14.
Falaye A.E., Ojo-Daniel H.A., Sule S.O., 2022. Effects of processing on Duckweed (Lemna minor) as fish feedstuff. Sci. Rep. Life Sci. 3, 53–67, https://doi.org/10.5281/zenodo....
15.
FAO, 1999. Duckweed - A tiny aquatic plant with enormous potential for agriculture and environment; FAO publications: Rome, Italy.
16.
Finno C.J., Valberg S.J., 2012. A comparative review of vitamin e and associated equine disorders. J. Vet. Intern. Med. 26, 1251–1266, https://doi.org/10.1111/j.1939....
17.
Fiordelmondo E., Ceschin S., Magi G.E., Mariotti F., Iaffaldano N., Galosi L., Roncarati A., 2022. Effects of partial substitution of conventional protein sources with duckweed (Lemna minor) meal in the feeding of rainbow trout (Oncorhynchus mykiss) on growth performances and the quality product. Plants 11, 1220, https://doi.org/10.3390/plants....
18.
Ge X., Zhang N., Phillips G.C., Xu J., 2012. Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol. Bioresour. Technol. 124, 485–488, http://dx.doi.org/10.1016/j.bi....
19.
Gwaze F.R., Mwale M., 2015. The prospect of duckweed in pig nutrition. A review. J. Agr. Sci. 7, 189–199, https://doi.org/10.5539/jas.v7....
20.
Hanczakowski P., Szymczyk B., Wawrzyński M., 1995. Composition and nutritive value of sewage-grown duckweed (Lemna minor L.) for rats. Anim. Feed Sci. Technol. 52, 339–343, https://doi.org/10.1016/0377-8....
21.
Hugue K.S., Chowdhury S.A., Kibra S.S.,1996. Study on the potentiality of duckweeds as a feed for cattle. Asian-Australas. J. Anim. Sci. 9, 133–137, https://doi.org/10.5713/ajas.1....
22.
Jahreis G., Schaefer U., 2011. Rapeseed (Brassica napus) oil and its benefits for human health. In: V. R. Preedy, R. R. Watson, and V. B. Patel (Eds.), Nuts & seeds in health and disease prevention (1st Ed.). Academic Press. London, Burlington, San Diego pp. 967–974, https:/doi.org/10.1016/B978-0-12-375688-6.10114-8
23.
Jahreis G., Brese M., Leiterer M., Schaefer U., Böhm V., 2016. Legume flours: Nutritionally important sources of protein and dietary fiber. Ernahrungs Umschau 63, 36–42, https://doi.org/10.4455/eu.201....
24.
Ifie I., Olatunde S., Ogbon O., Umukoro J.E., 2021. Processing techniques on phytochemical content, proximate composition, and toxic components in duckweed. Int. J. Veg. Sci., 27, 294–302, https://doi.org/10.1080/193152....
25.
Kaiser F., Harbach H., Schulz C., 2022. Rapeseed proteins as fishmeal alternatives: A review. Rev. Aquac. 14, 1887–1911, https://doi.org/10.1111/raq.12....
26.
Lagos L.V., Stein H.H., 2017. Chemical composition and amino acid digestibility of soybean meal produced in the United States, China, Argentina, Brazil, or India. J. Anim. Sci. 95, 1626–1636, https://doi.org/10.2527/jas201....
27.
Leng R. A., Stambolie J. H., Bell R., 1995. Duckweed – a potential high-protein feed resource for domestic animals and fish. Livest. Res. Rural. Develop. 7, 1, http://www.lrrd.org/lrrd7/1/3.....
28.
Li Y., Horsman M., Wang B., Wu N., Lan C.Q., 2008. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 81, 629–636, https://doi.org/10.1007/s00253....
29.
Lynch A., Kerry J.P., Buckley D.J., Morrissey P.A., Lopez-Bote C., 2001. Use of high pressure chromatography (HPLC) for the determination of-tocopherol levels on forage (silage/grass) samples collected from different regions in Ireland. Food Chem. 72, 521–524, https://doi.org/10.1016/S0308-....
30.
Mazen A.M.A, Zhang D., Franceschi V.R., 2003. Calcium oxalate formation in Lemna minor: physiological and ultrastructural aspects of high capacity calcium sequestration. New Phytol. 161, 435–448, https://doi.org/10.1111/j.1469....
31.
Mertens D.R., 2002. Gravimetric determination of amylase-treated neutral detergent fibre in feeds with refluxingbeakers or crucibles: collaborative study. J. Assoc. Off. Assoc. Chem. Int. 85, 1217–1240.
32.
Mohedano R.A., Rejane H.R., Costa R., Flávia A., Tavares A., Paulo B.F., 2012. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour. Technol. 112, 98–104, https://doi.org/10.1016/j.bior....
33.
Mwale M., Gwaze F.R., 2013. Characteristics of duckweed and its potential as feed source for chickens reared for meat production. A review. Sci. Res. Essays 8, 689–697, http://www.academicjournals.or....
34.
NRC, 2001. Nutrient Requirements of Dairy Cattle. 7th reviewed Edition. Nat. Acad. Press, Washington, DC.
35.
Ociepa-Kubicka A, Ociepa E., 2012. Toxic effects of heavy metals on plants, animals and humans (in Polish). Inżynieria i Ochrona Środowiska 12 , 169–180.
36.
Pagliuso D., Grandis A., Fortirer J.S., Camargo P., Floh E.I.S., Buckeridge M.S., 2022. Duckweeds as promising food feedstocks globally. Agronomy 12, 796, https://doi.org/10.3390/agrono....
37.
Polutchko S.K., Stewart J.J., McNamara M., Doherty Garci N., López-Pozo M., Adams W.W. III, Demmig-Adams B., 2022. Lemna as a sustainable, highly nutritious crop: nutrient production in different light environments. Nutraceuticals 2, 350–364, https://doi.org/10.3390/nutrac....
38.
Said D.S., Chrismadha T., Mayasari N., Widiyanto T., Ramandita A., 2022. Nutritional content and growth ability of duckweed spirodela polyrhiza on various culture media. In: IOP Conference Series: Earth and Environmental Science, 1062 (1), 012009, Https:/doi.org/10.1088/1755-1315/1062/1/012009.
39.
Shingfield K.J., Salo-Väänänen P., Pahkala E., Toivonen V., Jaakkola S., Piironen V., Huhtanen P., 2005. Effect of forage conservation method, concentrate level and propylene glycol on the fatty acid composition and vitamin content of cows’ milk. J. Dairy Res. 72, 349–361, https://doi.org/10.1017/S00220....
40.
Sońta M., Rekiel A., Batorska M., 2019. Use of duckweed (Lemna L.) in sustainable livestock production and aquaculture – a review. Ann. Anim. Sci. 19, 257–271, https://doi.org/10.2478/aoas-2....
41.
Sońta M., Łozicki A., Szymańska M., Sosulski T., Szara E., Wąs A., Gijs W.P. van Pruissen, Cornelissen R.L., 2020. Duckweed from a biorefinery system: Nutrient recovery efficiency and forage value. Energy 13, 5261, https://doi.org/10.3390/en1320....
42.
Spiegel M., Noordam M.Y., Fels-Klerx H.J., 2013. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Safety 12, 662–678, https://doi.org/10.1111/1541-4....
43.
Stewart J.J., Adams W.W. III, López-Pozo M., Doherty Garcia N., McNamara M., Escobar C.M., Demmig-Adams B., 2021. Features of the duckweed Lemna that support rapid growth under extremes of light intensity. Cells 10, 1481, https://doi.org/10.3390/cells1....
44.
Table of Chemical Composition and Nutritional Value of Domestic Feed, 2015. In Polish: Tabele składu chemicznego i wartości pokarmowej pasz krajowych. The National Research Institute of Animal Production, ISBN 978-83-7607-262-3, Balice, pp. 12–63.
45.
Tang J., Li Y., Ma J., Cheng J.J., 2015. Survey of duckweed diversity in Lake Chaoand total fatty acid, triacylglycerol, profiles of representative strains. Plant Biol. 17, 1066–1072, https://doi.org/10.1111/plb.12....
46.
Weiss W.P., 1998. Requirements of fat-soluble vitamins for dairy cows: A review. J. Dairy Sci. 81, 2493–2501, https://doi.org/10.3168/jds.S0....
47.
WHO, 2007. Joint FAO/WHO/UNU expert consultation on protein and amino acid requirements in human nutrition, 2002. Geneva, Switzerland.
48.
Verma R., Suthar S., 2014. Synchronized urban wastewater treatment and biomass production using duckweed Lemna gibba L. Ecol. Eng. 64, 337–343, http://doi.org/10.1016/j.ecole....
49.
Xiong Z., Liu L., Jian Z., Ma Y., Li H., Jin X., Liao B., Wang K., 2023. Vitamin E and multiple health outcomes: an umbrella review of meta-analyses. Nutrients 15, 3301, https://doi.org/10.3390/nu1515....
50.
Xu J., Cui W., Cheng J. J., Stomp A.M., 2011. Production of High-starch Duckweed and Its Conversion to Bioethanol. Biosyst. Eng. 110, 67–72, http://dx.doi.org/10.1016/j.bi....
51.
Xu J., Shen Y., Zheng Y., Smith G., Sun X.S., Wang D., Zhao Y., Zhang W., Li Y., 2021. Duckweed (Lemnaceae) for potentially nutritious human food: A review. Food Rev. Int. 39, 3620–3634, http://doi.org/10.1080/8755912....
52.
Yean Y., Candreva J., Shi H., Ernst E., Martienssen R., Schwender J., Shanklin J., 2013. Survey of the total fatty acid and triacylglycerol composition and content of 30 duckweed species and cloning of a Δ6-desaturase responsible for the production of γ-linolenic and stearidonic acids in Lemna gibba. BMC Plant Biol. 13, 201, http://doi.org/10.1186/1471-22....
53.
Zamri M.Z.A., Ramiah S.K., Jamein E.S., Zulkifli, I., Lokman I.H., Amirul F.M.A, Fadzlin S.A.A., S. Mochd Zamri, Jayanegara A., Hassim H.A., 2023. Potential use of black soldier fly, Hermetia illucens larvae in chicken feed as a protein replacer: a review J. Anim. Feed Sci, 32, 4, 341–356, https://doi.org/10.22358/jafs/....
54.
Zhang K.X., Zhang K.Y., Applegate T.J., Bai S.P., Ding X.M., Wang J.P., Peng H.W., Xuan Y., Su Z.W., Zeng Q.F., 2020. Evaluation of the standardized ileal digestibility of amino acids of rapeseed meals varying in protein solubility for Pekin ducks. Poult. Sci. 99, 1001–1009, https://doi.org/10.1016/j.psj.....
55.
Zhao X., Moates G.K., Wilson D.R., Ghogare R.J., Coleman M.J., Waldron K.W., 2015. Steam explosion pretreatment and enzymatic saccharification of duckweed (Lemna minor) biomass. Biomass Bioenergy 72, 206–215, https://doi.org/10.1016/j.biom....
CITATIONS (6):
1.
Duckweed-Based Optical Biosensor for Herbicide Toxicity Assessment
L.A.I. Ying-Jang, L.U. Pin-Cheng, Yi KUNG
Biosensors and Bioelectronics
L.A.I. Ying-Jang, L.U. Pin-Cheng, Yi KUNG
Biosensors and Bioelectronics
2.
Evaluation of the biochemical composition of the greater duckweed Spirodela polyrhiza (L. Schleiden)
R. Miltko, M. P. Majewska, N. Frączek, B. Kowalik
Journal of Animal and Feed Sciences
R. Miltko, M. P. Majewska, N. Frączek, B. Kowalik
Journal of Animal and Feed Sciences
3.
Duckweed: exploring its farm-to-fork potential for food production and biorefineries
Anim Ujong, Joncer Naibaho, Soudabeh Ghalamara, Brijesh K. Tiwari, Shay Hanon, Uma Tiwari
Sustainable Food Technology
Anim Ujong, Joncer Naibaho, Soudabeh Ghalamara, Brijesh K. Tiwari, Shay Hanon, Uma Tiwari
Sustainable Food Technology
4.
The effect of natural and synthetic zeolites on polysaccharidase activity in the rumen of Jersey heifers
M. P. Majewska, U. Wolska-Świętlicka, R. Miltko, G. Bełżecki, A. Kędzierska, B. Kowalik
Journal of Animal and Feed Sciences
M. P. Majewska, U. Wolska-Świętlicka, R. Miltko, G. Bełżecki, A. Kędzierska, B. Kowalik
Journal of Animal and Feed Sciences
5.
Duckweed extract-mediated green synthesis of ZnO nanoparticles and its antibacterial, antioxidant, and photocatalytic properties
Yogesh Kumar Shukla, Priyansh Pandey, Janardan Prasad Pandey, Alok Shukla, Jitendra Kumar
Biomass Conversion and Biorefinery
Yogesh Kumar Shukla, Priyansh Pandey, Janardan Prasad Pandey, Alok Shukla, Jitendra Kumar
Biomass Conversion and Biorefinery
6.
Lenteja de agua (Lemna minor): un potencial insumo proteico alternativo para la alimentación animal
Ronaldo Francesco Zevallos-Contreras, Walter Rolando Oscanoa-Condor
Revista Peruana de Investigación Agropecuaria
Ronaldo Francesco Zevallos-Contreras, Walter Rolando Oscanoa-Condor
Revista Peruana de Investigación Agropecuaria
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.