Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
REVIEW PAPER
Faecal biomarkers of gastrointestinal functionality in animals
1
ICAR-Indian Veterinary Research Institute, Faculty of Veterinary Science, Department of Animal Nutrition, 243122, Izatnagar, Bareilly, UP, India
2
Maharashtra Animal and Fishery Sciences University, Nagpur Veterinary College, Faculty of Animal Science, Department of Animal Nutrition, 440006, Nagpur, Maharashtra, India
These authors had equal contribution to this work
Publication date: 2024-02-12
Corresponding author
A. P. Dhok
Maharashtra Animal and Fishery Sciences University, Nagpur Veterinary College, Faculty of Animal Science, Department of Animal Nutrition, 440006, Nagpur, Maharashtra, India
Maharashtra Animal and Fishery Sciences University, Nagpur Veterinary College, Faculty of Animal Science, Department of Animal Nutrition, 440006, Nagpur, Maharashtra, India
J. Anim. Feed Sci. 2024;33(2):149-158
KEYWORDS
faecal biomarkergastrointestinal functionalityglucocorticoid metabolitesnon-invasiveorganic acid metabolites
TOPICS
ABSTRACT
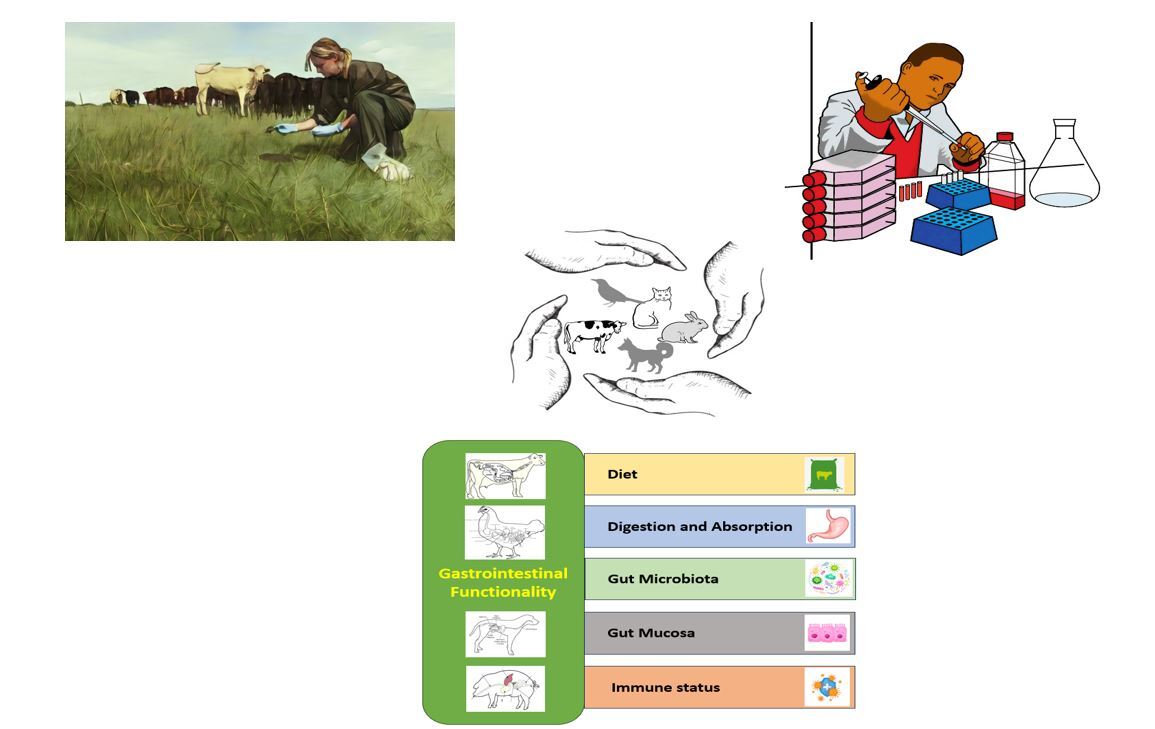
Effective functioning of the gastrointestinal (GI) tract is essential for
determining and sustaining animal health, welfare and performance. A balanced
diet, efficient digestion and absorption, normal and stable microbiota, a robust
immune system, and a healthy gut mucosa are the primary key components
determining optimal GI functionality. These pivotal factors offer an opportunity
to explore potential biomarkers for evaluating the performance of the GI
system in animals. Among various biological samples, faecal samples emerge
as advantageous due to their easy collection, non-invasiveness, applicability
in wild animals, minimum stress, and their contribution to animal welfare.
While literature on faecal biomarkers in animals is scarce, some studies have
investigated such indicators in monogastric, wild, and laboratory animals; these
include lactate and succinate (indicators of fermentative diarrhoea), sialic acid
(indicative of intestinal damage), glucocorticoid (stress marker), intestinal alkaline
phosphatase (associated with pathogenic intestinal damage), and lipocalin-2
and calprotectin (indicating intestinal inflammation). Faecal glucocorticoid
metabolites are particularly useful for evaluating environmental or captive stress
in wild animals. The aforementioned faecal biomarkers offer insights into events
influencing GI functionality and may pave the way for developing nutritional
interventions aimed at modulating the GI system to enhance animal welfare and
performance.
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
REFERENCES (74)
1.
AbellaV., Scotece M., Conde J., Gómez, R., Lois A., Pino, J., Gómez-Reino, J.J., Lago, F., Mobasheri, A., Gualillo, O., 2015. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 20, 565–571, https://doi.org/10.3109/135475....
2.
Alibrahim B., Aljasser M.I., Salh B., 2015. Fecal calprotectin use in inflammatory bowel disease and beyond: a mni-review. Can. J. Gastroenterol. Hepatol, 29, 157–163, https://doi.org/10.1155/2015/9....
3.
Allwin B., Jayathangaraj M.G., Palanivelrajan M., Raman M., 2016. Effect of endogenous faecal glucocorticoid metabolites on reproduction in wild pigs-a non-invasive approach. J. Vet. Sci. Technol. 7, 2, http://dx.doi.org/10.4172/2157....
4.
Barker S.B., Summerson W.H., 1941. The colorimetric determination of lactic acid in biological material. J. Biol. Chem. 138, 535–554, https://doi.org/10.1016/S0021-...
5.
Bischoff S.C., 2011. 'Gut health': a new objective in medicine? BMC Med. 9, 1–14, https://doi.org/10.1186/1741-7....
6.
Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M., 2014. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 14, 1–25, https://doi.org/10.1186/s12876...
7.
Broom L.J., Kogut M.H., 2018. Inflammation: friend or foe for animal production? Poult. Sci. 97, 510–514, https://doi.org/10.3382/ps/pex....
8.
Burdick N.C., Randel R.D., Carroll J.A., Welsh T.H., 2011. Interactions between temperament, stress, and immune function in cattle. Int. J Zool. 2011, https://doi.org/10.1155/2011/3....
9.
Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V., 2017. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technol. 234, 88–100, https://doi.org/10.1016/j.anif....
10.
Celi P., Verlhac V., Calvo E.P., Schmeisser J., Kluenter A.M., 2019. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed Sci. Technol. 250, 9–31, https://doi.org/10.1016/j.anif....
11.
Chakraborty S., Kaur S., Guha S., Batra S.K., 2012. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta Rev. Cancer. 1826, 129–169, https://doi.org/10.1016/j.bbca....
12.
Chassaing B., Srinivasan G., Delgado M.A., Young A.N., Gewirtz A.T., Vijay-Kumar M., 2012. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE, https://doi.org/10.1371/journa....
13.
Conway P.L., 1994. Function and regulation of the gastrointestinal microbiota of the pig. In: Souffrant W. and Hagemeister,H., Eds., Proceedings of the 6th International Symposium on Digestive Physiology in Pigs, 231–240.
14.
Cornick S., Tawiah A., Chadee K., 2015. Roles and regulation of the mucus barrier in the gut. Tissue Barriers, 3, 1–2, https://doi.org/10.4161/216883....
15.
Counotte G.H.M., Van't Klooster A.T., Van der Kuilen J., Prins R.A., 1979. An analysis of the buffer system in the rumen of dairy cattle. J. Anim. Sci. 49, 1536–1544, https://doi.org/10.2527/jas197....
16.
Csernus V., 1982. Antibodies of high affinity and specificity for radioimmunological determination of progesterone, testosterone, estradiol-17β and cortisol. Advances in steroid analysis. I. Akadémia Kiadó, 171–177.
17.
Dietert R.R., Silbergeld E.K., 2015. Biomarkers for the 21st century: listening to the microbiome. Toxicol. Sci. 144, 208–216, https://doi.org/10.1093/toxsci....
18.
Dohms J.E., Metz A., 1991. Stress – mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 30, 89–109, https://doi.org/10.1016/0165-2....
19.
Endris M., Feki E., 2021. Review on effect of stress on animal productivity and response of animal to stressors. J. Anim. Vet. Adv. 20, 1–14, http://dx.doi.org/10.36478/jav....
20.
Ewaschuk J.B., Naylor J.M., Palmer R., Whiting S.J., Zello G.A., 2004. D‐lactate production and excretion in diarrheic calves. J. Vet. Intern. Med, 18, 744–747, https://doi.org/10.1111/j.1939....
21.
Fawley J., Gourlay D.M., 2016. Intestinal alkaline phosphatase: a summary of its role in clinical disease. J. Surg. Res. 202, 225–234, https://doi.org/10.1016/j.jss.....
22.
Fernando P.S., Rose S.P., Mackenzie A.M., Silva S.S.P., 2011. Effect of diets containing potato protein or soya bean meal on the incidence of spontaneously-occurring subclinical necrotic enteritis and the physiological response in broiler chickens. Br. Poult. Sci. 52, 106–114, https://doi.org/10.1080/000716....
23.
Foell D., Frosch M., Sorg C., Roth J., 2004. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta. 344, 37–51, https://doi.org/10.1016/j.cccn....
24.
Heilmann R.M., Berghoff N., Mansell J. et al., 2018. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J. Vet. Intern. Med. 32, 679–692, https://doi.org/10.1111/jvim.1....
25.
Hernández J., Benedito J.L., Abuelo A., Castillo C., 2014. Ruminal acidosis in feedlot: from aetiology to prevention. Sci. World J., Article ID 702572, https://doi.org/10.1155/2014/7....
26.
Hollingsworth M.A., Swanson B.J., 2004. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60, https://doi.org/10.1038/nrc125....
27.
Hsieh H., Morin J., Filliettaz C., Varada R., LaBarre S., Radi Z., 2016. Fecal lipocalin-2 as a sensitive and noninvasive biomarker in the TNBS Crohn’s inflammatory bowel disease model. Toxicol. Pathol. 44, 1084–1094, https://doi.org/10.1177/019262....
28.
Huang Y.L., Chassard C., Hausmann M., Von Itzstein M., Hennet T., 2015. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat. Commun. 6, 8141, https://doi.org/10.1038/ncomms....
29.
Ji B., Nielsen J., 2015. From next-generation sequencing to systematic modeling of the gut microbiome. Front. Genet. 6, 219, https://doi.org/10.3389/fgene.....
30.
Jourdian G.W., Dean L., Roseman S., 1971. The sialic acids: XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 246, 430–435, https://doi.org/10.1016/S0021-....
31.
Judd T.A., Day A.S., Lemberg D.A., Turner D., Leach S.T., 2011. Update of fecal markers of inflammation in inflammatory bowel disease. J. Gastroenterol. Hepatol. 26, 1493–1499, https://doi.org/10.1111/j.1440....
32.
Jurkovich V., Kezer F.L., Ruff F., Bakony M., Kulcsár M., Kovács L., 2017. Heart rate, heart rate variability, faecal glucocorticoid metabolites and avoidance response of dairy cows before and after changeover to an automatic milking system. Acta Vet. Hung. 65, 301–313, https://doi.org/10.1556/004.20....
33.
Klasing K.C., 2007. Nutrition and the immune system. Br. Poult. Sci. 48, 525–537, https://doi.org/10.1080/000716....
34.
Kogut M.H., Arsenault R.J., 2016. Gut health: The new paradigm in food animal production. Front. Vet. Sci. 3, 71, https://doi.org/10.3389/fvets.....
35.
Lallès J.P., 2010. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 68, 323–332, https://doi.org/10.1111/j.1753....
36.
Lallès J.P., Fagerhol M.K., 2005. Faecal calprotectin: a non-invasive marker of inflammation in pigs. ISAH, 1, 405–408.
37.
Lee W.J., Hase K., 2014. Gut microbiota–generated metabolites in animal health and disease. Nat. Chem. Biol. 10, 416–424, https://doi.org/10.1038/nchemb....
38.
Lynch E.M., 2010. Characterisation of physiological and immune-related biomarkers of weaning stress in beef cattle, Doctoral dissertation, National University of Ireland Maynooth.
39.
Macfarlane G.T., Macfarlane S., 2007. Models for intestinal fermentation: association between food components, delivery systems, bioavailability and functional interactions in the gut. Curr. Opin. Biotechnol. 18, 156–162, https://doi.org/10.1016/j.copb....
40.
Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Thomas L.V., 2016. The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339, http://dx.doi.org/10.1136/gutj....
41.
Messmann S., Bagu E., Robia C., Palme R., 1999. Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. J. Vet. Med. A, 46, 621–631, https://doi.org/10.1046/j.1439....
42.
Millán J.L., 2006. Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic signalling 2, 335–341, https://doi.org/10.1007/s11302....
43.
Morrow C.J., Kolver E.S., Verkerk G.A., Matthews L.R., 2002. Fecal glucocorticoid metabolites as a measure of adrenal activity in dairy cattle. Gen. Comp. Endocrinol. 126, 229–241, https://doi.org/10.1006/gcen.2....
44.
Moussavi A.H., Gilbert R.O., Overton T.R., Bauman D.E., But W.R., 2007. Effects of feeding fish meal and n-3 fatty acids on ovarian and uterine responses in early lactating dairy cows. J. Dairy Sci. 90, 145–154, https://doi.org/10.3168/jds.S0....
45.
Niewold T.A., 2015. Intestinal health biomarkers in vivo. In: Intestinal health: Key to maximise growth performance in livestock. Chapter 9, p. 717–721, Wageningen Academic Publishers, https://doi.org/10.3920/978-90....
46.
Paduchova Z., Durackova Z., 2009. Fecal calprotectin as a promising marker of inflammatory diseases. Bratisl. Lek. Listy, 110, 598–602.
47.
Palme R., Möstl E., 1997. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Inst. J. Mammal Biol. 62, 192–197.
48.
Palme R., Robia C.H., Messmann S., Hofer J., Mostl E., 1999. Measurement of faecal cortisol metabolites in ruminants: a non-invasive parameter of adrenocortical function. Wien. Tierärztl. 86, 237–241.
49.
Phillips J.C., Ortega A., Cook M., Concepcion M., Kimmons T., Ralph K., Baldwin S., 2010. Activism and trust: Animal rights vs. animal welfare in the food supply chain. J. Food Distrib. Res. 41, 91–95, http://dx.doi.org/10.22004/ag.....
50.
Pluske J.R., 2013. Feed-and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 4, 1–7, https://doi.org/10.1186/2049-1....
51.
Radhakrishnan S., Reddivari L., Yu H., Knight R., Vanamala J., 2015. High‐calorie diet (HCD) induced elevation in calprotectin and lipocalin‐2 correlates with changes in gut bacteria and their metabolites in a porcine model. The FASEB J. 29, 913–13, https://doi.org/10.1096/fasebj....
52.
Rammes A., Roth J., Goebeler M., Klempt M., Hartmann M., Sorg C., 1997. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 272, 9496–9502, https://doi.org/10.1074/jbc.27....
53.
Rathore K.I., Berard J.L., Redensek A., Chierzi S., Lopez-Vales R., Santos M., Akira S., David S., 2011. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J. Neurosci. 31, 13412–13419, https://doi.org/10.1523/JNEURO....
54.
Rietschel E.T., Kirikae T., Schade F.U. et al., 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. The FASEB J. 8, 217–225, https://doi.org/10.1096/fasebj....
55.
Sapolsky R.M., Romero L.M., Munck A.U., 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89, https://doi.org/10.1210/edrv.2....
56.
Sato H., 2003. Fecal ammonia, urea, and organic acid levels in newborn dairy calves and their relations to diarrhea. J. JVMA 56, 517–521, https://doi.org/10.12935/jvma1....
57.
Sato H., Koiwa M., 2008. Fecal D‐and L‐lactate, succinate and volatile fatty acid levels, and relationships with fecal acidity and diarrhea in neonatal calves. Anim. Sci. J. 79, 187–192, https://doi.org/10.1111/j.1740....
58.
Sato H., Shiogama Y., 2009. Fecal excretion of alcohols and organic anions in neonatal dairy calves. Anim. Sci. J. 80, 171–175, https://doi.org/10.1111/j.1740....
59.
Shimomura Y., Sato H., 2006. Fecal D-and L-lactate, succinate, and volatile fatty acid levels in young dairy calves. J. Vet. Med. Sci. 68, 973–977, https://doi.org/10.1292/jvms.6....
60.
Starkey J.D., 2014. Triennial growth symposium – a role for vitamin D in skeletal muscle development and growth. J. Anim. Sci. 92, 887–892, https://doi.org/10.2527/jas.20....
61.
Taylor W., 1971. The excretion of steroid hormone metabolites in bile and feces. Vitam. Horm. 29, 201–285, https://doi.org/10.1016/S0083-....
62.
Thomas D.W., Henton D.H., 1985. The use of fecal alkaline phosphatase as an indicator of intestinal damage. Digestion 31, 82–88, https://doi.org/10.1159/000199....
63.
Tsukahara T., Ushida K., 2001. Organic acid profiles in feces of pigs with pathogenic or non-pathogenic diarrhea. J. Vet. Med. Sci. 63, 1351–1354, https://doi.org/10.1292/jvms.6....
64.
Vaos G., Kostakis I.D., Zavras N., Chatzemichael A., 2013. The role of calprotectin in pediatric disease. BioMed Res. Int., https://doi.org/10.1155/2013/5....
65.
Vermeire S., Van Assche G., Rutgeerts P., 2004. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 10, 661–665, https://doi.org/10.1097/000547....
66.
Vermeire S., Van Assche G., Rutgeerts P., 2005. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, .580–586, https://doi.org/10.1038/ncpgas....
67.
Vighi G., Marcucci F., Sensi L., Di Cara G., Frati F., 2008. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 153, 3–6, https://doi.org/10.1111/j.1365....
68.
Voganatsi A., Panyutich A., Miyasaki K.T., Murthy R.K., 2001. Mechanism of extracellular release of human neutrophil calprotectin complex. J. Leukoc. Biol. 70, 130–134, https://doi.org/10.1189/jlb.70....
69.
Wang Q., Li S., Tang X., Liang L., Wang F., Du H., 2019. Lipocalin 2 protects against Escherichia coli infection by modulating neutrophil and macrophage function. Front. Immunol. 10, 2594, https://doi.org/10.3389/fimmu.....
70.
Willing B.P., Van Kessel A.G., 2010. Host pathways for recognition: establishing gastrointestinal microbiota as relevant in animal health and nutrition. Livest. Sci. 133, 82–91, https://doi.org/10.1016/j.livs....
71.
Wrzecińska M., Czerniawska-Piątkowska E., Kowalczyk A., 2021. The impact of stress and selected environmental factors on cows’ reproduction. J. Appl. Anim. Res. 49, 318–323, https://doi.org/10.1080/097121....
72.
Xiao D., Wang Y., Liu G., He J., Qiu W., Hu X., Tang Z., 2014. Effects of chitosan on intestinal inflammation in weaned pigs challenged by enterotoxigenic Escherichia coli. PLoS One, 9, 104192, https://doi.org/10.1371/journa....
73.
Yang L., Francois F., Pei Z., 2012. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin. Cancer Res. 18, 2138–2144, https://doi.org/10.1158/1078-0....
74.
Yegani, M., Korver, D.R., 2008. Factors affecting intestinal health in poultry. Poult. Sci. 87, 2052–2063, https://doi.org/10.3382/ps.200....
CITATIONS (1):
1.
Effect of dietary wheat gluten levels on intestinal mucin flow and composition in young pigs
E. Święch
Journal of Animal and Feed Sciences
E. Święch
Journal of Animal and Feed Sciences
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.