Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
REVIEW PAPER
Metal nanoparticles – a real threat
or harmless companions for the thyroid gland? A review
1
The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Department of Animal Physiology,
Instytucka 3, 05-110 Jabłonna, Poland
Publication date: 2024-11-08
Corresponding author
A. Wójcik-Gładysz
The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Department of Animal Physiology, Instytucka 3, 05-110 Jabłonna, Poland
The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Department of Animal Physiology, Instytucka 3, 05-110 Jabłonna, Poland
J. Anim. Feed Sci. 2024;33(4):416-430
KEYWORDS
hypothalamic-pituitary-thyroid axismetal nanoparticlesreactive oxygen speciesthyroid hormonesthyroxinetriiodothyronine
TOPICS
ABSTRACT
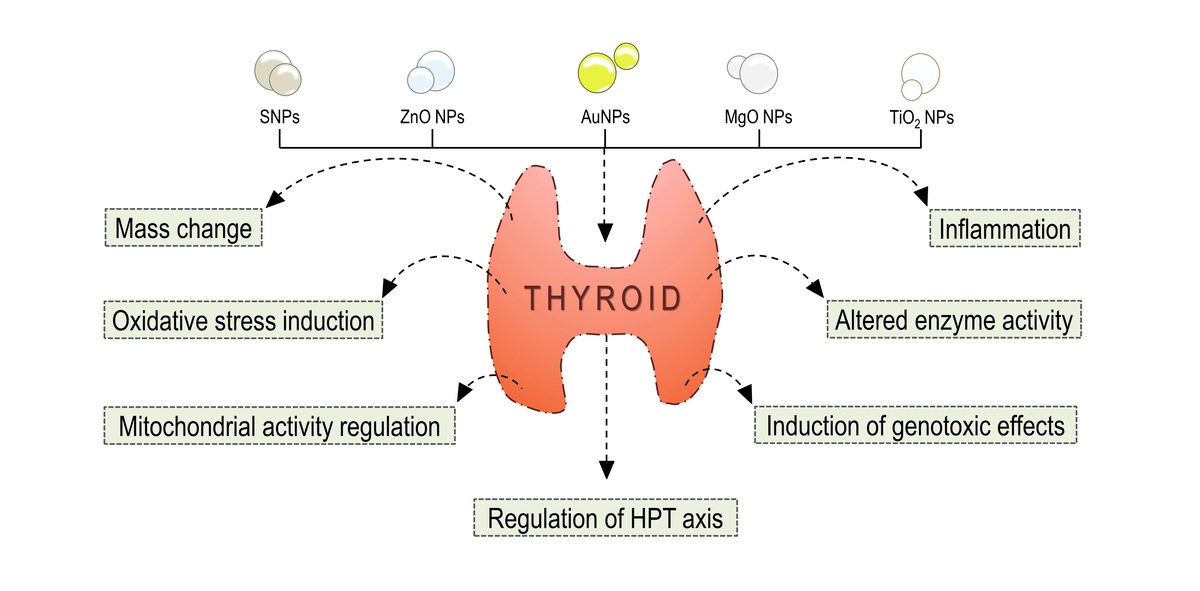
The application of metal nanoparticles (NPs) in various industries
is increasingly common due to their numerous beneficial properties. However,
recent studies have demonstrated that NPs can also pose significant
environmental risks, adversely affecting living organisms. The thyroid gland is
one of the organs whose functioning can be impaired by metal NPs. Evidence
from many studies indicates a possible toxic effect of metal NPs, mainly through
the activity of reactive oxygen species on the functioning of the endocrine
system, including the thyroid gland. Such disruptions may be manifested as
altered organ mass, induction of inflammation, oxidative stress, genotoxic
effects, and changes in the functioning of the hypothalamic-pituitary-thyroid axis.
Moreover, the large specific surface area of NPs can increase the bioavailability
of certain pollutants, thereby affecting the thyroid function of aquatic organisms.
It is essential to note that the potential toxicity of NPs is influenced by numerous
chemical and physical factors, and the resulting effects are also dependent
on the research model and methodology employed. This review highlights the
problem of the possible influence of NPs on thyroid gland function and the need
for further research in this area.
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
REFERENCES (92)
1.
Adewale O.B., Davids H., Cairncross L., Roux S., 2019. Toxicological behavior of gold nanoparticles on various models: influence of physicochemical properties and other factors. Int. J. Toxicol. 38, 357–384, https://doi.org/10.1177/109158....
2.
Akagi J.I., Mizuta Y., Akane H., Toyoda T., Ogawa K., 2023. Oral toxicological study of titanium dioxide nanoparticles with a crystallite diameter of 6 nm in rats. Part Fibre Toxicol. 20, 23, https://doi.org/10.1186/s12989....
3.
Al-Bishri W.M., 2018. Toxicity study of gold and silver nanoparticles on experimental animals. Pharmacophore 9, 48–55.
4.
Anjana G., Roy A., Rajeshkumar S., Ezhilarasan D., 2020. Cassia oleoresin mediated synthesis of magnesium oxide nanoparticles and brine shrimp lethality assay. J. Pharm. Res. Int. 32, 75–82, https://doi.org/10.9734/JPRI/2....
5.
Ashfaq F., Hayee S., Basheer Z., Naseem A., Akhtar N., Nadeem A., 2023. Study of ZnO Nanoparticles on Thyroid Hormones, Testosterone Level and Testes Histology. Pakistan Journal of Emerging Science and Technologies 3, 1–9, https://doi.org/10.58619/pjest....
6.
Averill-Bates D., 2024. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta. Mol. Cell. Res. 1871, 119573, https://doi.org/10.1016/j.bbam....
7.
Bianco A.C., da Conceição R.R., 2018. The deiodinase trio and thyroid hormone signaling. Methods Mol. Biol. 1801, 67–83, https://doi.org/10.1007/978-1-....
8.
Blaser S.A., Scheringer M., Macleod M., Hungerbühler K., 2008. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 390, 396–409, https://doi.org/10.1016/j.scit....
9.
Brent G.A., 2012. Mechanisms of thyroid hormone action. J. Clin. Invest. 122, 3035–3042, https://doi.org/10.1172/JCI600....
10.
Carew A.C., Hoque M.E., Metcalfe C.D., Peyrot C., Wilkinson K.J., Helbing C.C., 2015. Chronic sublethal exposure to silver nanoparticles disrupts thyroid hormone signaling during Xenopus laevis metamorphosis. Aquat. Toxicol. 159, 99–108, https://doi.org/10.1016/j.aqua....
11.
Chen J., Wang H., Long W. et al., 2013. Sex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in mice. Int. J. Nanomedicine 8, 2409–2419, https://doi.org/10.2147/IJN.S4....
12.
Cornu R., Béduneau A., Martin H., 2022. Ingestion of titanium dioxide nanoparticles: a definite health risk for consumers and their progeny. Arch. Toxicol. 96, 2655–2686, https://doi.org/10.1007/s00204....
13.
Dayem A.A., Hossain M.K., Lee S.B., Kim K., Saha S.K., Yang G.M., Choi H.Y., Cho S.G., 2017. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 18, 120, https://doi.org/10.3390/ijms18....
14.
de Oliveira I.M., Cavallin M.D., Corrêa D.E.D.C., Razera A., Mariano D.D., Ferreira F., Romano M.A., Marino Romano R., 2020. Proteomic profiles of thyroid gland and gene expression of the hypothalamic-pituitary-thyroid axis are modulated by exposure to AgNPs during prepubertal rat stages. Chem. Res. Toxicol. 33, 2605–2622, https://doi.org/10.1021/acs.ch....
15.
El-Kady M.M., Ansari I., Arora C., Rai N., Soni S., Verma D.K., Singh P., Mahmoud A.E.D., 2023. Nanomaterials: a comprehensive review of applications, toxicity, impact, and fate to environment. J. Mol. Liq. 370, 121046, https://doi.org/10.1016/j.moll....
16.
Elshama S.S., Abdallah M.E., Abdel-Karim R.I., 2018. Zinc oxide nanoparticles: therapeutic benefits and toxicological hazards. Open Nanomed. J. 5, 16–22, https://doi.org/10.2174/187593....
17.
Espanani H.R., Fazilati M., Sadeghi L., Yousefi B.V., Bakhshiani S., Amraie E., 2013. Investigation of the Zinc Oxide nanoparticle’s effect on sex hormones and cholesterol in rat. Int. Res. J. Biological Sci. 2, 54–58.
18.
Ferdous Z., Nemmar A., 2020. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 21, 2375, https://doi.org/10.3390/ijms21....
19.
Fong P.P., Thompson L.B., Carfagno G.L., Sitton A.J., 2016. Long-term exposure to gold nanoparticles accelerates larval metamorphosis without affecting mass in wood frogs (Lithobates sylvaticus) at environmentally relevant concentrations. Environ. Toxicol. Chem. 35, 2304–2310, https://doi.org/10.1002/etc.33....
20.
Fujihara J., Nishimoto N., 2024. Review of zinc oxide nanoparticles: toxicokinetics, tissue distribution for various exposure routes, toxicological effects, toxicity mechanism in mammals, and an approach for toxicity reduction. Biol. Trace Elem. Res. 202, 9–23, https://doi.org/10.1007/s12011....
21.
Gavrila A., Hollenberg A.N., 2019. The hypothalamic-pituitary-thyroid axis: physiological and clinical implications. In: Luster M., Duntas L.H., Wartofsky L. The thyroid and its diseases. A comprehensive guide for the clinician. Springer International Publishing, Switzerland, pp. 13–23, https://doi.org/10.1007/978-3-....
22.
Ge L., Li Q., Wang M., Ouyang J., Li X., Xing M.M., 2014. Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int. J. Nanomedicine 9, 2399–2407, https://doi.org/10.2147/IJN.S5....
23.
Gelli K., Porika M., Anreddy R.N., 2015. Assessment of pulmonary toxicity of MgO nanoparticles in rats. Environ. Toxicol 30, 308-314, https://doi.org/10.1002/tox.21....
24.
Ghosh Chaudhuri R., Paria S., 2012. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 112, 2373–2433, https://doi.org/10.1021/cr1004....
25.
Guo Y., Chen L., Wu J., Hua J., Yang L., Wang Q., Zhang W., Lee J.S., Zhou B., 2019. Parental co-exposure to bisphenol A and nano-TiO2 causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring. Sci. Total. Environ. 650, 557–565, https://doi.org/10.1016/j.scit....
26.
Hage M., Zantout M.S., Azar S.T., 2011. Thyroid disorders and diabetes mellitus. J. Thyroid Res. 2011, 439463, https://doi.org/10.4061/2011/4....
27.
Hammami I., Alabdallah N.M., Al Jomaa A., Kamoun M., 2021. Gold nanoparticles: synthesis properties and applications. J. King Saud Univ. Sci. 33, 101560, https://doi.org/10.1016/j.jksu....
28.
Hammond S.A., Carew A.C., Helbing C.C., 2013. Evaluation of the effects of titanium dioxide nanoparticles on cultured Rana catesbeiana tailfin tissue. Front. Genet. 4, 251, https://doi.org/10.3389/fgene.....
29.
Hinther A., Vawda S., Skirrow R., Veldhoen N., Collins P., Cullen J.T., van Aggelen G.C., Helbing C.C., 2010. Nanomaterials induce stress and alter thyroid hormone action in amphibia at or below American water quality guidelines. Environ. Sci. Technol. 44, 8314–8321, https://doi.org/10.1021/es1019....
30.
Hoermann R., Midgley J.E., Larisch R., Dietrich J.W., 2016. Relational stability in the expression of normality, variation, and control of the thyroid function. Front. Endocrinol. 7:142, https://doi.org/10.3389/fendo.....
31.
Hong F., Wang L., 2018. Nanosized titanium dioxide-induced premature ovarian failure is associated with abnormalities in serum parameters in female mice. Int. J. Nanomedicine 13, 2543–2549, https://doi.org/10.2147/IJN.S1....
32.
Hou J., Wu Y., Li X., Wei B., Li S., Wang X., 2018. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 193, 852–860, https://doi.org/10.1016/j.chem....
33.
Ibrahim N., Sabic E., Wakwak M., El-Wardany I., El-Homosany Y., El-Deen Mohammad N., 2020. In-ovo and dietary supplementation of selenium nano-particles influence physiological responses, immunological status and performance of broiler chicks. J. Anim. Feed Sci. 29, 46–58, https://doi.org/10.22358/jafs/....
34.
Katarzyńska-Banasik D., Grzesiak M., Kowalik K., Sechman A., 2021. Administration of silver nanoparticles affects ovarian steroidogenesis and may influence thyroid hormone metabolism in hens (Gallus domesticus). Ecotoxicol. Environ. Saf. 208, 111427, https://doi.org/10.1016/j.ecoe....
35.
Katarzyńska-Banasik D., Kowalik K., Sechman A., 2024. Influence of silver nanoparticles on mRNA expression of thyroid hormone-related genes in the thyroid gland and liver of laying hens. Domest. Anim. Endocrinol. 86, 106820, https://doi.org/10.1016/j.doma....
36.
Kim K.T., Eo M.Y., Nguyen T.T.H., Kim S.M., 2019. General review of titanium toxicity. Int. J. Implant. Dent. 5, 10, https://doi.org/10.1186/s40729....
37.
Kirkland A.E., Sarlo G.L., Holton K.F., 2018. The Role of Magnesium in Neurological Disorders. Nutrients 10, 730, https://doi.org/10.3390/nu1006....
38.
Kirkland D., Aardema M.J., Battersby R.V. et al., 2022. A weight of evidence review of the genotoxicity of titanium dioxide (TiO2). Regul. Toxicol. Pharmacol. 136, 105263, https://doi.org/10.1016/j.yrtp....
39.
Król A., Pomastowski P., Rafińska K., Railean-Plugaru V., Buszewski B., 2017. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 249, 37–52, https://doi.org/10.1016/j.cis.....
40.
Kulak E., Ognik K., Stępniowska A., Sembratowicz I., 2018. The effect of administration of silver nanoparticles on silver accumulation in tissues and the immune and antioxidant status of chickens. J. Anim. Feed Sci. 27, 44–54, https://doi.org/10.22358/jafs/....
41.
Lazim F.G., Luaibi N.M., Muhammed H.J., 2021. Effect of ZnO on thyroid function and evaluation of the levels of TSH receptor gene in thyroid tissue of female rat. Biochem. Cell. Arch. 21, 1027-1033.
42.
Lei L., Qiao K., Guo Y., Han J., Zhou B., 2020. Titanium dioxide nanoparticles enhanced thyroid endocrine disruption of pentachlorophenol rather than neurobehavioral defects in zebrafish larvae. Chemosphere 249, 126536, https://doi.org/10.1016/j.chem....
43.
Lepionka T., Białek M., Czauderna M., Szlis M., Białek A., 2021. Lipidomic Profile and Enzymes Activity in Hepatic Microsomes of Rats in Physiological and Pathological Conditions. Int. J. Mol. Sci. 23, 442, https://doi.org/10.3390/ijms23....
44.
Luaibi N.M., Mohammed R.A., 2023. Physiological and Hormonal Effects of Titanium Dioxide Nanoparticles on Thyroid and Kidney Functions. Baghdad Sci. J. 20, 767–777, https://doi.org/10.21123/bsj.2....
45.
Luaibi N.M., Zayed N. A., 2020. Investigation of the zinc oxide nanoparticles effect on thyroid and testosterone hormones in male rats. Cihan Univ.-Erbil Sci. J. 4, 26–31, https://doi.org/10.24086/cuesj....
46.
Luo Z., Li Z., Xie Z., Sokolova I.M., Song L., Peijnenburg W.J.G.M., Hu M., Wang Y., 2020. Rethinking nano-TiO2 safety: overview of toxic effects in humans and aquatic animals. Small 16, e2002019, https://doi.org/10.1002/smll.2....
47.
Majeed S., Danish M., Muhadi N.F.B.B., 2018. Genotoxicity and apoptotic activity of biologically synthesized magnesium oxide nanoparticles against human lung cancer A-549 cell line. Adv. Nat. Sci.: Nanosci. Nanotechnol. 9, 025011, https://doi.org/10.1088/2043-6....
48.
Mangalampalli B., Dumala N., Perumalla Venkata R., Grover P., 2018. Genotoxicity, biochemical, and biodistribution studies of magnesium oxide nano and microparticles in albino wistar rats after 28-day repeated oral exposure. Environ. Toxicol. 33, 396–410, https://doi.org/10.1002/tox.22....
49.
Martínez G., Merinero M., Pérez-Aranda M., Pérez-Soriano E.M., Ortiz T., Villamor E., Begines B., Alcudia A., 2021. Environmental impact of nanoparticles' application as an emerging technology: a review. Materials 14, 166, https://doi.org/10.3390/ma1401....
50.
McShan D., Ray P.C., Yu H., 2014. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 22, 116–127, https://doi.org/10.1016/j.jfda....
51.
Mekuye B., Abera B., 2023. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select 4, 486–501, https://doi.org/10.1002/nano.2....
52.
Miao W., Zhu B., Xiao X., Li Y., Dirbaba N.B., Zhou B., Wu H., 2015. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 161, 117–126, https://doi.org/10.1016/j.aqua....
53.
Mohammed R.S., Aadim K.A., Ahmed K.A., 2022. Estimation of in vivo toxicity of MgO/ZnO core/shell nanoparticles synthesized by eco-friendly non-thermal plasma technology. Appl. Nanosci. 12, 3783–3795, https://doi.org/10.1007/s13204....
54.
Moncayo R., Moncayo H., 2014. The WOMED model of benign thyroid disease: acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 3, 44–64, https://doi.org/10.1016/j.bbac....
55.
Nappi A., Murolo M., Cicatiello A.G., Sagliocchi S., Di Cicco E., Raia M., Stornaiuolo M., Dentice M., Miro C., 2022. Thyroid hormone receptor isoforms alpha and beta play convergent roles in muscle physiology and metabolic regulation. Metabolites 12, 405, https://doi.org/10.3390/metabo....
56.
Niżnik Ł., Noga M., Kobylarz D., Frydrych A., Krośniak A., Kapka-Skrzypczak L., Jurowski K., 2024. Gold nanoparticles (AuNPs)-toxicity, safety and green synthesis: a critical review. Int. J. Mol. Sci. 25, 4057, https://doi.org/10.3390/ijms25....
57.
Obaid A.H., Abbas S.A., Hussein F.M., Jawad A.A., Ibrahim T.A. Obaeed N. H., 2022. Investigation of the magnesium oxide nanoparticles effect on thyroid (T3, T4) thyroid stimulating hormones (TSH) in rat. Al-Nahrain J. Sci. 25, 20–24, https://doi.org/10.22401/ANJS.....
58.
Ortiga-Carvalho T.M., Chiamolera M.I., Pazos-Moura C.C., Wondisford F.E., 2016. Hypothalamus-pituitary-thyroid axis. Compr. Physiol. 6, 1387–1428, https://doi.org/10.1002/cphy.c....
59.
Osmond-McLeod M.J., Oytam Y., Kirby J.K., Gomez-Fernandez L., Baxter B., McCall M.J., 2014. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology 8 Suppl 1, 72–84, https://doi.org/10.3109/174353....
60.
Parang Z., Davood M., 2019. Synthesis of silver nano-particles by electrochemical method and the effects on the serum levels of thyroid hormones (T3, T4) in adult male rats. Iran. J. Toxicol. 13, 21–25, https://doi.org/10.32598/IJT.1....
61.
Pham P.C., Pham P.A., Pham S.V., Pham P.T., Pham P.M., Pham P.T., 2014. Hypomagnesemia: a clinical perspective. Int. J. Nephrol. Renovasc. Dis. 7, 219–230, https://doi.org/10.2147/IJNRD.....
62.
Piantanida E., Ippolito S., Gallo D. et al., 2020. The interplay between thyroid and liver: implications for clinical practice. J. Endocrinol. Invest. 43, 885–899, https://doi.org/10.1007/s40618....
63.
Pickford D.B., 2010. Screening chemicals for thyroid-disrupting activity: A critical comparison of mammalian and amphibian models. Crit. Rev. Toxicol. 40, 845–892, https://doi.org/10.3109/104084....
64.
Sabella S., Galeone A., Vecchio G., Cingolani R., Pompa P.P., 2011. AuNPs are toxic in vitro and in vivo: a review. J. Nanosci. Lett. 1, 145–165.
65.
Saberi A, Baltatu MS, Vizureanu P., 2024. Recent advances in magnesium-magnesium oxide nanoparticle composites for biomedical applications. Bioengineering (Basel) 11, 508, https://doi.org/10.3390/bioeng....
66.
Sakr S., Steenkamp V., 2021. Zinc oxide nanoparticles induce oxidative stress and histopathological toxicity in the thyroid gland and liver of rats. Toxicol. Environ. Chem. 103, 399–422, https://doi.org/10.1080/027722....
67.
Saleh A.A., El-Magd M.A., 2018. Beneficial effects of dietary silver nanoparticles and silver nitrate on broiler nutrition. Environ. Sci. Pollut. Res. Int. 25, 27031–27038, https://doi.org/10.1007/s11356....
68.
Saleh T.A., 2020. Nanomaterials: classification, properties, and environmental toxicities. Environ. Technol. Inno. 20, 101067, https://doi.org/10.1016/j.eti.....
69.
Samy A., Hassan H.M.A., Elsherif H.M.R., 2022. Effect of nano oxide and traditional zinc (oxide and sulphate) sources on performance, bone characteristics and physiological parameters of broiler chicks. Int. J. Vet. Sci. 11, 486–492, https://doi.org/10.47278/journ....
70.
Sani A., Cao C., Cui D., 2021. Toxicity of gold nanoparticles (AuNPs): a review. Biochem. Biophys. Rep. 26, 100991, https://doi.org/10.1016/j.bbre....
71.
Sharma V., Singh S.K., Anderson D., Tobin D.J., Dhawan A., 2011. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J. Nanosci. Nanotechnol. 11, 3782–3788, https://doi.org/10.1166/jnn.20....
72.
Shaukat A., Hussain G., Irfan S., Ijaz M.U., Anwar H., 2022. Therapeutic Potential of MgO and MnO Nanoparticles Within the Context of Thyroid Profile and Pancreatic Histology in a Diabetic Rat Model. Dose-Response 20, 15593258221128743, https://doi.org/10.1177/155932....
73.
Sruthi S., Ashtami J., Mohanan P.V., 2018. Biomedical application and hidden toxicity of zinc oxide nanoparticles. Mater. Today Chem. 10, 175–186, https://doi.org/10.1016/j.mtch....
74.
Stathatos N., 2019. Anatomy and Physiology of the Thyroid Gland. In: Luster M., Duntas L.H., Wartofsky L. The thyroid and its diseases. A comprehensive guide for the clinician. Springer International Publishing, Switzerland, pp. 3–12, https://doi.org/10.1007/978-3-....
75.
Sulaiman A.A., Luaibi N.M., Qassim H.A., 2018. Effects of silver nanoparticles on thyroid gland structure and function in female rats. Asian J. Pharm. Clin. Res. 11, 509, https://doi.org/10.22159/ajpcr....
76.
Tassinari R., Cubadda F., Moracci G. et al., 2014. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology 8, 654–662, https://doi.org/10.3109/174353....
77.
Unuane D., Velkeniers B., 2020. Impact of thyroid disease on fertility and assisted conception. Best Pract. Res. Clin. Endocrinol. Metab. 34, 101378. https://doi.org/10.1016/j.beem....
78.
Verma S.K., Nisha K., Panda P.K., Patel P., Kumari P., Mallick M.A., Sarkar B., Das B., 2020. Green synthesized MgO nanoparticles infer biocompatibility by reducing in vivo molecular nanotoxicity in embryonic zebrafish through arginine interaction elicited apoptosis. Sci. Total Environ. 713, 136521, https://doi.org/10.1016/j.scit....
79.
Vidal-Cevallos P., Murúa-Beltrán Gall S., Uribe M., Chávez-Tapia N.C., 2023. Understanding the relationship between nonalcoholic fatty liver disease and thyroid disease. Int. J. Mol. Sci. 24, 14605, https://doi.org/10.3390/ijms24....
80.
Wang Q., Chen Q., Zhou P., Li W., Wang J., Huang C., Wang X., Lin K., Zhou B., 2014. Bioconcentration and metabolism of BDE-209 in the presence of titanium dioxide nanoparticles and impact on the thyroid endocrine system and neuronal development in zebrafish larvae. Nanotoxicology. 8 Suppl 1, 196–207, https://doi.org/10.3109/174353....
81.
Weber A.G., Birk B., Müller C., Schneider S., van Ravenzwaay B., Funk-Weyer D., Landsiedel R., 2022. The thyroid hormone converting enzyme human deiodinase 1 is inhibited by gold ions from inorganic salts, organic substances, and by small-size nanoparticles. Chem. Biol. Interact. 351, 109709, https://doi.org/10.1016/j.cbi.....
82.
Wypych A., Ożgo M., Bernaciak M. et al., 2024. Effect of feeding high fat diets differing in fatty acid composition on oxidative stress markers and protein expression in mouse kidney. J. Anim. Feed Sci. 33, 170–184, https://doi.org/10.22358/jafs/....
83.
Yang J., Wang X., Khan M.R., Hammouda G.A., Alam P., Meng L., Zhang Z., Zhang W., 2024. New opportunities and advances in magnesium oxide (MgO) nanoparticles in biopolymeric food packaging films. Sustain. Mater. Techno. 40, e00976, https://doi.org/10.1016/j.susm....
84.
Yang S., Lian G., 2020. ROS and diseases: role in metabolism and energy supply. Mol. Cell. Biochem. 467, 1-12, https://doi.org/10.1007/s11010....
85.
Younes M., Aquilina G., Castle L. et al., 2021. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 19, e06585, https://doi.org/10.2903/j.efsa....
86.
Yousef M.I., Al-Hamadani M.Y.I., Kamel M.A., 2019. Reproductive toxicity of aluminum oxide nanoparticles and zinc oxide nanoparticles in male rats. Nanoparticle 1, 3, https://doi.org/10.35702/nano.....
87.
Zhang J., Wang F., Yalamarty S.S.K., Filipczak N., Jin Y., Li X., 2022. Nano silver-induced toxicity and associated mechanisms. Int. J. Nanomedicine 17, 1851–1864, https://doi.org/10.2147/IJN.S3....
88.
Zhang L., Ren Y., Li Y., Meng Y., Fang H., Yang L., 2023a. Regulation of Nod-like receptor expression in the liver of ewes during early pregnancy. J. Anim. Feed Sci. 32, 267–279, https://doi.org/10.22358/jafs/....
89.
Zhang X., Song Y., Gong H., Wu C., Wang B., Chen W., Hu J., Xiang H., Zhang K., Sun M., 2023b. Neurotoxicity of titanium dioxide nanoparticles: a comprehensive review. Int. J. Nanomedicine 18, 7183–7204, https://doi.org/10.2147/IJN.S4....
90.
Zhou Q., Xue S., Zhang L., Chen G., 2022. Trace elements and the thyroid. Front. Endocrinol. 13, 904889, https://doi.org/10.3389/fendo.....
91.
Ziental D., Czarczynska-Goslinska B., Mlynarczyk D.T., Glowacka-Sobotta A., Stanisz B., Goslinski T., Sobotta L., 2020. Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials (Basel) 10, 387, https://doi.org/10.3390/nano10....
92.
Zoeller R.T., Brown T.R., Doan L.L., Gore A.C., Skakkebaek N.E., Soto A.M., Woodruff T.J., Vom Saal F.S., 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153, 4097–4110, https://doi.org/10.1210/en.201....
CITATIONS (1):
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.