Journal IF and Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
ORIGINAL PAPER
Regulation of Nod-like receptor expression in the liver of ewes
during early pregnancy
1
Hebei University of Engineering, School of Life Sciences and Food Engineering, No. 19 Taiji Road, Handan 056038, China
Publication date: 2023-04-20
J. Anim. Feed Sci. 2023;32(3):267-279
KEYWORDS
TOPICS
ABSTRACT
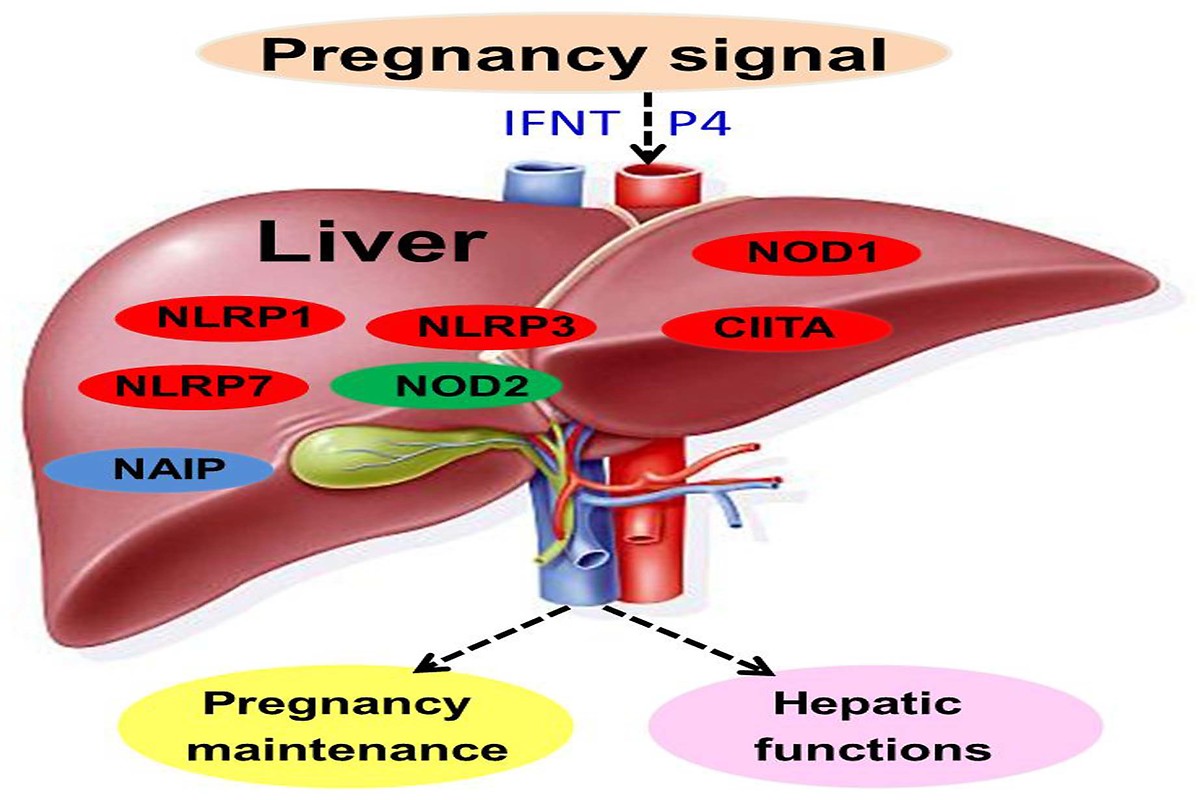
Multiple factors are involved in the regulation of maternal-foetal
tolerance, and nucleotide-binding and oligomerisation domain (NOD)-like
receptors (NLRs) were shown to play roles in innate immune signalling
pathways. The objective of this study was to investigate the effects of early
pregnancy on the expression of NLRs in the liver of ewes. Livers from ewes
were collected on day 16 of the oestrous cycle, and days 13, 16 and 25 of
gestation (n = 6 for each group). Real-time quantitative PCR, Western blot
and immunohistochemical analysis were used to analyse mRNA and protein
expression of NLRs, including NOD1, NOD2, major histocompatibility complex
class II transactivator (CIITA), neuronal apoptosis inhibitor protein (NAIP), NLR
family, pyrin domain-containing 1 (NLRP1), NLRP3 and NACHT, LRR and PYD
domains-containing protein 7 (NLRP7). The data showed that NOD1, CIITA,
NLRP1, NLRP3 and NLRP7 were upregulated in maternal liver on days 13 to
25 of pregnancy, and NOD2 expression was upregulated on days 13 and 16
of pregnancy, but downregulated on day 25 of pregnancy. In addition, NOD2
and NLRP7 proteins were located in the endothelial cells of the proper hepatic
arteries and portal veins, and in hepatocytes. NAIP expression was the highest
at day 16 of pregnancy. In conclusion, this study reported for the first time that
early pregnancy modulated the expression of NLRs, suggesting that NLRs were
involved in the regulation of maternal hepatic immune functions during early
pregnancy in sheep.
FUNDING
This work was supported by the grants from
the Natural Science Foundation of Hebei Province,
China (C2022402038), and Hebei Science and
Technology Agency, China (22326602D).
CONFLICT OF INTEREST
The Authors declare that there is no conflict of
interest.
REFERENCES (71)
1.
Abi Nahed R., Elkhoury Mikhael M., Reynaud D. et al., 2022. Role of NLRP7 in normal and malignant trophoblast cells. Biomedicines 10, 252, https://doi.org/10.3390/biomed...
2.
Abi Nahed R., Reynaud D., Borg A.J. et al., 2019. NLRP7 is increased in human idiopathic fetal growth restriction and plays a critical role in trophoblast differentiation. J. Mol. Med. 97, 355–367, https://doi.org/10.1007/s00109...
3.
Abu-Raya B., Michalski C., Sadarangani M., Lavoie P.M., 2020. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 11, 575197, https://doi.org/10.3389/fimmu....
4.
Bai J., Zhang L., Zhao Z., Li N., Wang B., Yang L., 2020. Expression of melatonin receptors and CD4 in the ovine thymus, lymph node, spleen and liver during early pregnancy. Immunology 160, 52–63, https://doi.org/10.1111/imm.13...
5.
Bartlett A.Q., Vesco K.K., Purnell J.Q., et al., 2021. Pregnancy and weaning regulate human maternal liver size and function. Proc. Natl. Acad. Sci. U.S.A. 118, e2107269118, https://doi.org/10.1073/pnas.2...
6.
Body-Malapel M., Dharancy S., Berrebi D. et al., 2008. NOD2: a potential target for regulating liver injury. Lab. Invest. 88, 318–327, https://doi.org/10.1038/labinv...
7.
Bonney E.A., 2017. Alternative theories: pregnancy and immune tolerance. J. Reprod. Immunol. 123, 65–71, https://doi.org/10.1016/j.jri....
8.
Boyle J.P., Parkhouse R., Monie T.P., 2014. Insights into the molecular basis of the NOD2 signalling pathway. Open Biol. 4, 140178, https://doi.org/10.1098/rsob.1...
9.
Bremer L., Schramm C., Tiegs G., 2016. Immunology of hepatic diseases during pregnancy. Semin. Immunopathol. 38, 669–685, https://doi.org/10.1007/s00281...
10.
Cao N., Cao L., Gao M., Wang H., Zhang L., Yang L., 2021. Changes in mRNA and protein levels of gonadotropin releasing hormone and receptor in ovine thymus, lymph node, spleen, and liver during early pregnancy. Domest. Anim. Endocrinol. 76, 106607, https://doi.org/10.1016/j.doma...
11.
Caruso R., Warner N., Inohara N., Núñez G., 2014. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41, 898–908, https://doi.org/10.1016/j.immu...
12.
Costello M.J., Joyce S.K., Abrahams V.M., 2007. NOD protein expression and function in first trimester trophoblast cells. Am. J. Reprod. Immunol. 57, 67–80, https://doi.org/10.1111/j.1600...
13.
Devaiah B.N., Singer D.S., 2013. CIITA and its dual roles in MHC gene transcription. Front. Immunol. 4, 476, https://doi.org/10.3389/fimmu....
14.
Diez E., Yaraghi Z., MacKenzie A., Gros P., 2000. The neuronal apoptosis inhibitory protein (Naip) is expressed in macrophages and is modulated after phagocytosis and during intracellular infection with Legionella pneumophila. J. Immunol. 164, 1470–1477, https://doi.org/10.4049/jimmun...
15.
Fang S., Zhang T., Qiao H., Hao S., Zhang L., Yang L., 2022. Expression of nuclear factor kappa B components in the ovine maternal liver in early pregnancy periods. Anim. Sci. J. 93, e13724, https://doi.org/10.1111/asj.13...
16.
Feng P., Wu J., Ren Y., Zhang L., Cao J., Yang L., 2022. Early pregnancy regulates the expression of prolactin and its receptor in the thymus, the liver, the spleen and lymph nodes in sheep. Domest. Anim. Endocrinol. 81, 106731, https://doi.org/10.1016/j.doma...
17.
Feng P., Yang G., Zhang W., Zhang L., Wu J., Yang L., 2021. Early pregnancy regulates expression of complement components in ovine liver. Anim. Sci. J. 92, e13660, https://doi.org/10.1111/asj.13...
18.
Fernandes F.P., Leal V.N.C., de Lima D.S., Reis E.C., Pontillo A., 2020. Inflammasome genetics and complex diseases: a comprehensive review. Eur. J. Hum. Genet. 28, 1307–1321, https://doi.org/10.1038/s41431...
19.
Gao M., Cai C., Han X., Wang L., Zhang W., Zhang L., Yang L., 2021. The early stage of pregnancy modulates toll-like receptor signaling in the ovine liver. J. Appl. Anim. Res. 49, 374–381, https://doi.org/10.1080/097121...
20.
Haneklaus M., O'Neill L.A.J., 2015. NLRP3 at the interface of metabolism and inflammation. Immunol. Rev. 265, 53–62, https://doi.org/10.1111/imr.12...
21.
Huang J.Y., Yu P.H., Li Y.C., Kuo P.L., 2017. NLRP7 contributes to in vitro decidualization of endometrial stromal cells. Reprod. Biol. Endocrinol. 15, 66, https://doi.org/10.1186/s12958...
22.
Huntington G.B., 1990. Energy metabolism in the digestive tract and liver of cattle: influence of physiological state and nutrition. Reprod. Nutr. Dev. 30, 35–47, https://doi.org/10.1051/rnd:19...
23.
Ingram-Crooks J., Holcik M., Drmanic S., MacKenzie A.E., 2002. Distinct expression of neuronal apoptosis inhibitory protein (NAIP) during murine development. Neuroreport 13, 397–402, https://doi.org/10.1097/000017...
24.
Jahn G.A., Edery M., Belair L., Kelly P.A., Djiane J., 1991. Prolactin receptor gene expression in rat mammary gland and liver during pregnancy and lactation. Endocrinology 128, 2976–2984, https://doi.org/10.1210/endo-1...
25.
Ka H., Hunt J.S., 2003. Temporal and spatial patterns of expression of inhibitors of apoptosis in human placentas. Am. J. Pathol. 163, 413–322, https://doi.org/10.1016/S0002-...
26.
Kandil D., Leiman G., Allegretta M., Trotman W., Pantanowitz L., Goulart R., Evans M., 2007. Glypican-3 immunocytochemistry in liver fine-needle aspirates: a novel stain to assist in the differentiation of benign and malignant liver lesions. Cancer Cytopathol. 111, 316–322, https://doi.org/10.1002/cncr.2...
27.
Kany S., Horstmann J.P., Sturm R., Mörs K., Relja B., 2018. Reduced NLRP3 gene expression limits the IL-1β cleavage via inflammasome in monocytes from severely injured trauma patients. Mediat. Inflamm. 2018, 1752836, https://doi.org/10.1155/2018/1...
28.
Kay C., Wang R., Kirkby M., Man S.M., 2020. Molecular mechanisms activating the NAIP-NLRC4 inflammasome: implications in infectious disease, autoinflammation, and cancer. Immunol. Rev. 297, 67–82, https://doi.org/10.1111/imr.12...
29.
Koch K.S., Leffert H.L., 2011. Ectopic expression of CD74 in Ikkβ-deleted mouse hepatocytes. Acta Histochem. 113, 428–435, https://doi.org/10.1016/j.acth...
30.
Livak K.J., Schmittgen T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408, https://doi.org/10.1006/meth.2...
31.
Murphy A.J., Kraakman M.J., Kammoun H.L., et al., 2016. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 23, 155–164, https://doi.org/10.1016/j.cmet...
32.
Nagatomo H., Akizawa H., Sada A., et al., 2015. Comparing spatial expression dynamics of bovine blastocyst under three different procedures: in-vivo, in-vitro derived, and somatic cell nuclear transfer embryos. Jpn. J. Vet. Res. 63, 159–171, https://doi.org/10.14943/jjvr....
33.
Oda T., Nakamura R., Kasamatsu T., Gotoh N., Okuda K., Saitoh T., Handa H., Murakami H., Yamashita T., 2022. DNA-double strand breaks enhance the expression of major histocompatibility complex class II through the ATM-NF-κΒ-IRF1-CIITA pathway. Cancer Gene Ther. 29, 225–240, https://doi.org/10.1038/s41417...
34.
Ott T.L., 2020. Immunological detection of pregnancy: Evidence for systemic immune modulation during early pregnancy in ruminants. Theriogenology 150, 498–503, https://doi.org/10.1016/j.ther...
35.
Rauch I., Deets K.A., Ji D.X., et al., 2017. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46, 649–659, https://doi.org/10.1016/j.immu...
36.
Relja B., Horstmann J.P., Kontradowitz K., Jurida K., Schaible A., Neunaber C., Oppermann E., Marzi I., 2015. Nlrp1 inflammasome is downregulated in trauma patients. J. Mol. Med. 93, 1391–400, https://doi.org/10.1007/s00109...
37.
Reynaud D., Abi Nahed R., Lemaitre N., et al., 2021. NLRP7 promotes choriocarcinoma growth and progression through the establishment of an immunosuppressive microenvironment. Cancers 13, 2999, https://doi.org/10.3390/cancer...
38.
Rocha C.C., da Silveira J.C., Forde N., Binelli M., Pugliesi G., 2021. Conceptus-modulated innate immune function during early pregnancy in ruminants: a review. Anim. Reprod. 18, e20200048, https://doi.org/10.1590/1984-3...
39.
Rosato R., Lindenbergh-Kortleve D., Neck J., Drop S., Jahn G., 2002. Effect of chronic thyroxine treatment on IGF-I, IGF-II and IGF-binding protein expression in mammary gland and liver during pregnancy and early lactation in rats. Eur. J. Endocrinol. 146, 729–739, https://doi.org/10.1530/eje.0....
40.
Ryu B.J., Han J.W., Kim R.H., Yun S., Kim T.H., Hur S.E., Kim C.J., Lee S.K., 2017. Activation of NOD-1/JNK/IL-8 signal axis in decidual stromal cells facilitates trophoblast invasion. Am. J. Reprod. Immunol. 78, e12672, https://doi.org/10.1111/aji.12...
41.
Scott M.J., Chen C., Sun Q., Billiar T.R., 2010. Hepatocytes express functional NOD1 and NOD2 receptors: a role for NOD1 in hepatocyte CC and CXC chemokine production. J. Hepatol. 53, 693–701, https://doi.org/10.1016/j.jhep...
42.
Silva A.P.C., Costa E.A., Macêdo A.A., Martins T.M., Borges A.M., Paixão T.A., Santos R.L., 2012. Transcription of pattern recognition receptors and abortive agents induced chemokines in the bovine pregnant uterus. Vet. Immunol. Immunopathol. 145, 248–256, https://doi.org/10.1016/j.veti...
43.
Soczewski E., Grasso E., Gallino L., Hauk V., Fernández L., Gori S., Paparini D., Leirós C.P., Ramhorst R., 2020. Immunoregulation of the decidualization program: focus on the endoplasmic reticulum stress. Reproduction 159, R203–R211, https://doi.org/10.1530/REP-19...
44.
Stødle G.S., Silva G.B., Tangerås L.H., et al., 2018. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin. Exp. Immunol. 193, 84–94, https://doi.org/10.1111/cei.13...
45.
Strauss J.F. 3rd, Romero R., Gomez-Lopez N., Haymond-Thornburg H., Modi B.P., Teves M.E, Pearson L.N., York T.P., Schenkein H.A., 2018. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am. J. Obstet. Gynecol. 218, 294–314.e2, https://doi.org/10.1016/j.ajog...
46.
Taabazuing C.Y., Griswold A.R., Bachovchin D.A., 2020. The NLRP1 and CARD8 inflammasomes. Immunol. Rev. 297, 13–25, https://doi.org/10.1111/imr.12...
47.
Tian X., Pascal G., Monget P., 2009. Evolution and functional divergence of NLRP genes in mammalian reproductive systems. BMC Evol. Biol. 9, 202, https://doi.org/10.1186/1471-2...
48.
Tsai P.Y., Chen K.R., Li Y.C., Kuo P.L., 2019. NLRP7 is involved in the differentiation of the decidual macrophages. Int. J. Mol. Sci. 20, 5994, https://doi.org/10.3390/ijms20...
49.
Velázquez M.M.L., Peralta M.B., Angeli E., Stassi A.F., Gareis N.C., Durante L., Cainelli S., Salvetti N.R., Rey F., Ortega H.H., 2019. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 206, 1–10, https://doi.org/10.1016/j.anir...
50.
Wang L., Hartmann P., Haimerl M., Bathena S.P., Sjöwall C., Almer S., Alnouti Y., Hofmann A.F., Schnabl B., 2014. Nod2 deficiency protects mice from cholestatic liver disease by increasing renal excretion of bile acids. J. Hepatol. 60, 1259–1267, https://doi.org/10.1016/j.jhep...
51.
Wang Y., Han X., Zhang L., Cao N., Cao L., Yang L., 2019. Early pregnancy induces expression of STAT1, OAS1 and CXCL10 in ovine spleen. Animals 9, 882, https://doi.org/10.3390/ani911...
52.
Wei J., Chen Q., James J.L., Stone P.R., Chamley L.W., 2015. IL-1 beta but not the NALP3 inflammasome is an important determinant of endothelial cell responses to necrotic/dangerous trophoblastic debris. Placenta 36, 1385–1392, https://doi.org/10.1016/j.plac...
53.
Yang L., Bai J., Zhao Z., Li N., Wang Y., Zhang L., 2019a. Differentialexpression of T helper cytokines in the liver during early pregnancy in sheep. Anim. Reprod. 16, 332–339, https://doi.org/10.21451/1984-...
54.
Yang L., Guo R., Yao X., Yan J., Bai Y., Zhang L., 2018a. Expression of progesterone receptor and progesterone-induced blocking factor in the spleen during early pregnancy in ewes. Livest. Sci. 209, 14–19, https://doi.org/10.1016/j.livs...
55.
Yang L., Han X., Zhang L., Li N., Zhao Z., Bai J., 2020a. Changes in expression of prostaglandin synthase in ovine liver during early pregnancy. Can. J. Anim. Sci. 100, 432–439, https://doi.org/10.1139/CJAS-2...
56.
Yang L., Li N., Zhang L., Bai J., Zhao Z., Wang Y., 2020b. Effects of early pregnancy on expression of interferon-stimulated gene 15, STAT1, OAS1, MX1, and IP-10 in ovine liver. Anim. Sci. J. 91, e13378, https://doi.org/10.1111/asj.13...
57.
Yang L., Liu Y., Lv W., Wang P., Wang B., Xue J., Zhang L., 2018b. Expression of interferon-stimulated gene 15-kDa protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim. Sci. J. 89, 1540–1548, https://doi.org/10.1111/asj.13...
58.
Yang L., Liu B., Yan X., Zhang L., Gao F., Liu Z., 2017a. Expression of ISG15 in bone marrow during early pregnancy in ewes. Kafkas Univ. Vet. Fak. Derg. 23, 767–772, https://doi.org/10.9775/kvfd.2...
59.
Yang L., Wang Q., Liu Y., Zhang L., Lv W., Liu B., 2019b. Expression profiles of interferon-stimulated gene 15 and prostaglandin synthases in the ovine lymph nodes during early pregnancy. Mol. Reprod. Dev. 86, 100–108, https://doi.org/10.1002/mrd.23...
60.
Yang L., Zang S., Bai Y., Yao X., Zhang L., 2017b. Effect of early pregnancy on the expression of progesterone receptor and progesterone-induced blocking factor in ovine lymph node. Theriogenology 93, 78–83, https://doi.org/10.1016/j.ther...
61.
Yoo I., Kim D., Han J., Lee S., Hong M., Jeon B.Y., Kim J.M., Ka H., 2020. Transcriptomic analysis of interferon-γ-regulated genes in endometrial explants and their possible role in regulating maternal endometrial immunity during the implantation period in pigs, a true epitheliochorial placentation species. Theriogenology 155, 114–124, https://doi.org/10.1016/j.ther...
62.
Zangara M.T., Johnston I., Johnson E.E., McDonald C., 2021. Mediators of metabolism: an unconventional role for NOD1 and NOD2. Int. J. Mol. Sci. 22, 1156, https://doi.org/10.3390/ijms22...
63.
Zhang L., Cao L., Yang F., Han X., Wang Y., Cao N., Yang L., 2020. Relative abundance of interferon-stimulated genes STAT1, OAS1, CXCL10 and MX1 in ovine lymph nodes during early pregnancy. Anim. Reprod. Sci. 214, 106285, https://doi.org/10.1016/j.anir...
64.
Zhang L., Li Y., Zhao Z., Cai J., Zhao S., Yang L., 2022. Modulation of nod-like receptor expression in the thymus during early pregnancy in ewes. Vaccines 10, 2128, https://doi.org/10.3390/vaccin...
65.
Zhang L., Xue J., Wang Q., Lv W., Mi H., Liu Y., Yang L., 2018. Changes in expression of ISG15, progesterone receptor and progesterone-induced blocking factor in ovine thymus during early pregnancy. Theriogenology 121, 153–159, https://doi.org/10.1016/j.ther...
66.
Zhang L., Zhao Z., Wang Y., Li N., Cao N., Yang L., 2020. Changes in expression of interferon-stimulated genes and ubiquitin activating enzyme E1-like in ovine thymus during early pregnancy. Anim. Reprod. 17, e20190134, https://doi.org/10.1590/1984-3...
67.
Zhang L.Y., Mi H., Yan J.K., Yan X.X., Yang L., 2017. Pregnancy-associated changes in expression of progesterone receptor and progesterone-induced blocking factor genes in bone marrow of ewes. Anim. Reprod. Sci. 186, 77–84, https://doi.org/10.1016/j.anir...
68.
Zhang Y., Zhang Y., Li C., Fu S., Yang C., Song Y., Liu M., Wang Z., Liang P., Zhang J., 2019. NOD1 modulates decidual stromal cell function to maintain pregnancy in the early trimester. Cell Biochem. Funct. 37, 464–473, https://doi.org/10.1002/cbf.34...
69.
Zhao L., Liang X., Ma Y., Li J., Liao S., Chen J., Wang C., 2020. AK002210 promotes the proliferation, migration and invasion of trophoblast cell through regulating miR-590/NAIP signal axis. Arch. Biochem. Biophys. 688, 108366, https://doi.org/10.1016/j.abb....
70.
Zhao Z., Li Y., Cao J., Fang H., Zhang L., Yang L., 2022. Early pregnancy modulates expression of Nod-like receptor family in lymph nodes of ewes. Animals 12, 3285, https://doi.org/10.3390/ani122...
71.
Zheng C., 2021. The emerging roles of NOD-like receptors in antiviral innate immune signaling pathways. Int. J. Biol. Macromol. 169, 407–413, https://doi.org/10.1016/j.ijbi...
CITATIONS (6):
1.
Maternal hepatic immunology during pregnancy
Ling Yang, Yao Meng, Yuxiang Shi, Hongxu Fang, Leying Zhang
Frontiers in Immunology
Ling Yang, Yao Meng, Yuxiang Shi, Hongxu Fang, Leying Zhang
Frontiers in Immunology
2.
Effects of early pregnancy on NOD-like receptor expression in the ovine endometrium
Leying Zhang, Jiabao Cai, Xinxin Wang, Zhen Yang, Haiquan Ding, Ling Yang
Frontiers in Veterinary Science
Leying Zhang, Jiabao Cai, Xinxin Wang, Zhen Yang, Haiquan Ding, Ling Yang
Frontiers in Veterinary Science
3.
Modulation of PI3K/AKT/mTOR signaling pathway in the ovine liver and duodenum during early pregnancy
Hongxu Fang, Xinxin Wang, Zhongyue Wang, Xiaoxin Ma, Leying Zhang, Ling Yang
Domestic Animal Endocrinology
Hongxu Fang, Xinxin Wang, Zhongyue Wang, Xiaoxin Ma, Leying Zhang, Ling Yang
Domestic Animal Endocrinology
4.
Metal nanoparticles – a real threat
or harmless companions for the thyroid gland? A review
K. Wysoczańska, D. Tomaszewska-Zaremba, A. Wójcik-Gładysz
Journal of Animal and Feed Sciences
K. Wysoczańska, D. Tomaszewska-Zaremba, A. Wójcik-Gładysz
Journal of Animal and Feed Sciences
5.
Amperometric sensing of prostate cancer biomarker (Sarcosine) using HiPIMS deposited nickel nitride films-decorated zinc oxide nanorod heterostructures
Nishchal Pardhi, Wei-Chun Cheng, Sheng-Chi Chen, Hui Sun, Mani Govindasamy
Applied Surface Science Advances
Nishchal Pardhi, Wei-Chun Cheng, Sheng-Chi Chen, Hui Sun, Mani Govindasamy
Applied Surface Science Advances
6.
Integrating traditional Chinese pulse diagnosis with machine learning: novel approaches for pregnancy and coronary heart disease identification
Meng Qiao, Zhaoshuai Yan, Chaoren Tan, Chao Lei, Wenxi Peng, Xingguang Geng, Xinghua Xiang, Wei Yang, Yitao Zhang, Zhifei Wang
Scientific Reports
Meng Qiao, Zhaoshuai Yan, Chaoren Tan, Chao Lei, Wenxi Peng, Xingguang Geng, Xinghua Xiang, Wei Yang, Yitao Zhang, Zhifei Wang
Scientific Reports
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.