Current issue
Online first
Archive
About the Journal
Editorial Office
Editorial Board
Copy right and self-archiving policy
Peer review process
Instructions for Reviewers
Printed version subscription
Abstracting and indexing
Contact
Instructions for Authors
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
Article publication charges
Policies
General information
Open Access, Licensing terms, Commercial use and Copyright terms policies
Self-archiving policy and Archive policies
Article Correction and Withdrawal policy
Manuscript Submission policy
Authorship policy
Conflict of Interest policy
Language considerations policy
Plagiarism and Duplicate publications policy
Ethics policy
Review process policy
Acceptance of manuscripts policy
Online First Articles and Special Issues policies
Generative artificial intelligence (AI) policy
Advertising policy
REVIEW PAPER
The role and molecular mechanisms of copper in regulating
animal lipid metabolism
1
Shandong Agricultural University, College of Animal Science and Technology, Ministry of Agriculture and Rural Affairs,
Key Laboratory of Efficient Utilization of Non-grain Feed Resources (Co-constructions by Ministry and Province),
271017 Tai’an, China
2
Sichuan Agricultural University, Institute of Animal Nutrition, Key Laboratory of Animal Disease-resistant,
611130 Chengdu, China
3
Research Center of Guangdong Haid Group Co., Ltd., 511400 Guangzhou, China
These authors had equal contribution to this work
Publication date: 2024-11-08
Corresponding author
Y. Li
Shandong Agricultural University, College of Animal Science and Technology, Ministry of Agriculture and Rural Affairs, Key Laboratory of Efficient Utilization of Non-grain Feed Resources (Co-constructions by Ministry and Province), 271017 Tai’an, China
Shandong Agricultural University, College of Animal Science and Technology, Ministry of Agriculture and Rural Affairs, Key Laboratory of Efficient Utilization of Non-grain Feed Resources (Co-constructions by Ministry and Province), 271017 Tai’an, China
J. Anim. Feed Sci. 2024;33(4):401-415
KEYWORDS
TOPICS
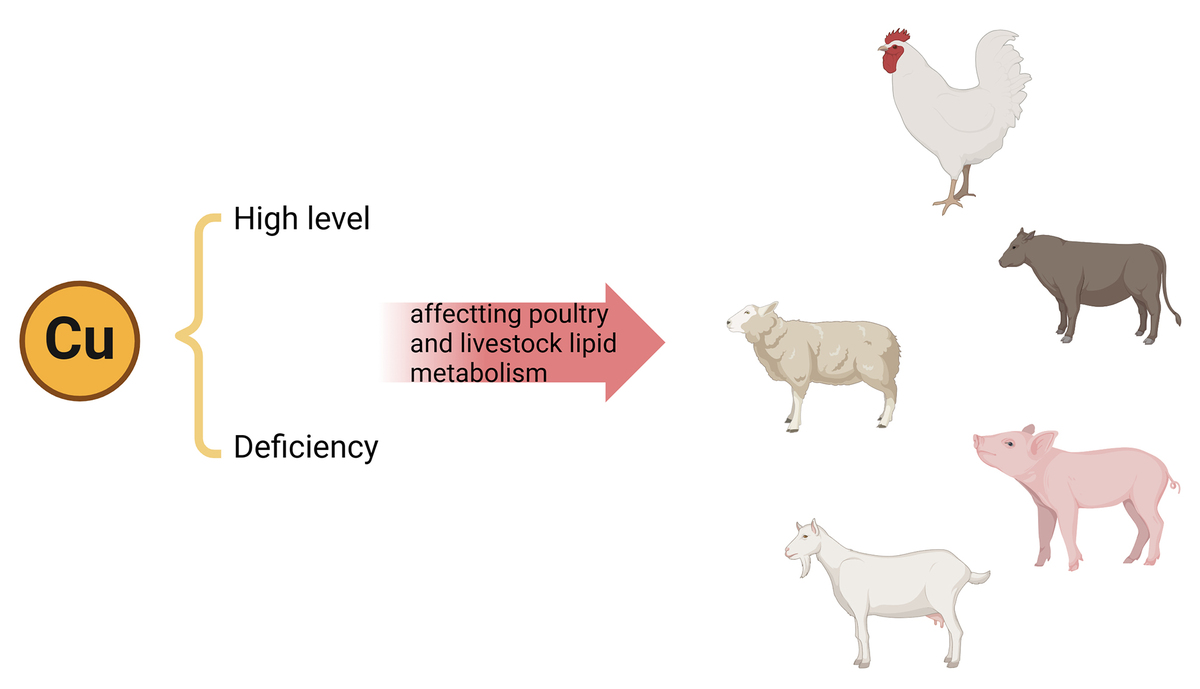
ABSTRACT
Copper serves as a crucial nutrient in animal organisms, acting
as a cofactor in metabolic processes and facilitating enzyme formation.
Research indicates that high copper levels can enhance animal production
performance. However, excessive copper intake can lead to its accumulation,
causing metabolic disruptions linked to various diseases. Moreover, copper
supplementation can effectively reduce lipid synthesis, especially cholesterol
and triglycerides, while enhancing fat oxidation and breakdown by regulating
the activity of ATPase copper transporting beta (ATP7B), cyclic adenosine
monophosphate (cAMP) levels and endoplasmic reticulum stress, thereby
decreasing lipid accumulation in vivo. Additionally, copper deficiency in humans
has been closely associated with diseases characterised by fat accumulation.
Thus, this article comprehensively outlines the intricate interplay between copper
and lipid metabolism, elucidating potential underlying mechanisms.
FUNDING
This research was supported by the National Key Research and Development Program of China (2023YFD1300803), the Natural Science Foundation of Shandong Province (ZR2022QC084) and the National Natural Science Foundation of China (32302784).
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
REFERENCES (137)
1.
Aigner E., Strasser M., Haufe H., et al., 2010. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 105, 1978–1985, https://doi.org/10.1038/ajg.20....
2.
Allen K.G.D., Klevay L.M., 1978. Cholesterolemia and cardiovascular abnormalities in rats caused by copper deficiency. Atherosclerosis 29, 81–93, https://doi.org/10.1016/0021-9....
3.
Arredondo M., Uauy R., González M., 2000. Regulation of copper uptake and transport in intestinal cell monolayers by acute and chronic copper exposure. Biochim. Biophys. Acta BBA - Gen. Subj. 1474, 169–176, https://doi.org/10.1016/S0304-....
4.
Bakalli R.I., Pesti G.M., Ragland W.L., Konjufca V., 1995. Dietary copper in excess of nutritional requirement reduces plasma and breast muscle cholesterol of chickens1,2. Poult. Sci. 74, 360–365, https://doi.org/10.3382/ps.074....
5.
Barthel A., Ostrakhovitch E.A., Walter P.L., Kampkötter A., Klotz L.-O., 2007. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: Mechanisms and consequences. Arch. Biochem. Biophys. 463, 175–182, https://doi.org/10.1016/j.abb.....
6.
Basseri S., Austin R.C., 2011. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem. Res. Int. 2012, e841362, https://doi.org/10.1155/2012/8....
7.
Blavi L., Solà D., Monteiro A., Pérez J.F., Stein H.H., 2021. Inclusion of dicopper oxide instead of copper sulfate in diets for growing-finishing pigs results in greater final body weight and bone mineralization, but reduced accumulation of copper in the liver. J. Anim. Sci. 99, skab127, https://doi.org/10.1093/jas/sk....
8.
Borgese N., Francolini M., Snapp E., 2006. Endoplasmic reticulum architecture: structures in flux. Curr. Opin. Cell Biol. 18, 358–364, https://doi.org/10.1016/j.ceb.....
9.
Braga C.B.M., Ferreira I.M. de L., Marchini J.S., Cunha S.F. de C. da, 2015. Copper and magnesium deficiencies in patints with short bowel syndrome receiving parenteral nutrition or oral feedin. Arq. Gastroenterol. 52, 94–99, https://doi.org/10.1590/S0004-....
10.
Cao H., Guo J., Su R., Pan J., Li Y., Tang Z., 2010. Effects of dietary copper levels from different sources on liver injury of broilers. Chin. J. Anim. Nutr., 22, 39–45, https://doi.org/10.3969/j.issn....
11.
Chen X., Cai Q., Liang R., et al., 2023. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 14, 1–12, https://doi.org/10.1038/s41419....
12.
Chen G.H., Luo Z., Hogstrand C., Wu K., Ling S.C., 2018. SREBP1, PPARG and AMPK pathways mediated the Cu-induced change in intestinal lipogenesis and lipid transport of yellow catfish Pelteobagrus fulvidraco. Food Chem. 269, 595–602, https://doi.org/10.1016/j.food....
13.
Chen C., Zhou Q., Yang R., et al., 2021. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011-2014). Ecotoxicol. Environment. Safety. 218, 112295, https://doi.org/10.1016/j.ecoe....
14.
Cheng J., Fan C., Zhang W., Yan X., Wang L., Jia Z., Zhu X., 2010. Effects of dietary copper source and level on metabolic hormones and lipogenic and lipolytic enzyme activities in lambs. Small Rumin. Res. 89, 12–17, https://doi.org/10.1016/j.smal....
15.
Clarkson A.H., Paine S., Martín-Tereso J., Kendall N.R., 2020. Copper physiology in ruminants: trafficking of systemic copper, adaptations to variation in nutritional supply and thiomolybdate challenge. Nutr. Res. Rev. 33, 43–49, https://doi.org/10.1017/S09544....
16.
Collins J.F., 2021. Chapter Nine - Copper nutrition and biochemistry and human (patho)physiology. In: N.A.M. Eskin (Editor).. Advances in Food and Nutrition Research. Academic Press. Florida (US), https://doi.org/10.1016/bs.afn....
17.
Correa L.B., Zanetti M.A., Del Claro G.R., de Melo M.P., Rosa A.F., Saran Netto A., 2012. Effect of supplementation of two sources and two levels of copper on lipid metabolism in Nellore beef cattle. Meat Sci. 91, 466–471, https://doi.org/10.1016/j.meat....
18.
Cui H., Xu Z., Peng X., Zhu K., Deng J., 2010. The effect of high copper on lymphocyte apoptosis of lymphoid organs in chicken. Acta Vet. Zootech Sin. 38, 601–607.
19.
Czech A., Wlazło Ł., Łukaszewicz M., Florek M., Nowakowicz-Dębek B., 2023. Fermented rapeseed meal enhances the digestibility of protein and macro-and microminerals and improves the performance of weaner pigs. Anim. Feed Sci. Tech. 300, 115656, https://doi.org/10.1016/j.anif....
20.
Dalto D.B., Audet I., Roy C., Deschêne K., Villeneuve G., Matte J.J., Lapointe J., 2023. Effects of dietary zinc/copper ratios on the metabolism of zinc and copper in weaned pigs. J. Anim. Sci. 101, https://doi.org/10.1093/jas/sk....
21.
Daniel J.-B., Brugger D., van der Drift S., van der Merw D., Kendall N., Windisch W., Doelman J., Martín-Tereso J., 2023. Zinc, Copper, and manganese homeostasis and potential trace metal accumulation in dairy cows: longitudinal study from late lactation to subsequent mid-lactation. J. Nutr. 153, 1008–1018, https://doi.org/10.1016/j.tjnu....
22.
Daval M., Foufelle F., Ferré P., 2006. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 574, 55–62, https://doi.org/10.1113/jphysi....
23.
De Feyter S., Beyens A., Callewaert B., 2023. ATP7A-related copper transport disorders: A systematic review and definition of the clinical subtypes. J. Inherit. Metab. Dis. 46, 163–173, https://doi.org/10.1002/jimd.1....
24.
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP)., 2016. Revision of the currently authorised maximum copper content in complete feed. EFSA J. 14, e04563, https://doi.org/10.2903/j.efsa....
25.
Elgerwi A., Pistl J., Bires J., Klikova K., 1999. The influence of industrial intoxication with copper on selected parameters of cellular immunity in sheep. Vet. Med. - UZPI Czech Repub. 44, 171–176, https://agris.fao.org/search/e....
26.
Engle T. E., Spears J. W., Xi L., Edens F. W., 2000. Dietary copper effects on lipid metabolism and circulating catecholamine concentrations in finishing steers. J. Anim. Sci. 78, 2737–2744, https://doi.org/10.2527/2000.7....
27.
Felix K., Nagel W., Hartmann H. J., Weser U., 1990. Copper transfer through the intestinal wall Serosal release of metallothionein. Bio. Met. 3, 141–145, https://doi.org/10.1007/BF0117....
28.
Felix T. L., Weiss W. P., Fluharty, F. L., Loerch S. C., 2012. Effects of copper supplementation on feedlot performance, carcass characteristics, and rumen sulfur metabolism of growing cattle fed diets containing 60% dried distillers grains. J. Anim. Sci. 90(8), 2710-2716, https://doi.org/10.2527/jas.20....
29.
Feng C., Xie B., Wuren Q., Gao M., 2020. Meta-analysis of the correlation between dietary copper supply and broiler performance. PloS one. 15(5), e0232876, https://doi.org/10.1371/journa....
30.
Fields M., Ferretti R. J., Smith J. C., Reiser S., 1983. Effect of copper deficiency on metabolism and mortality in rats fed sucrose or starch diets. J. Nutr. 113, 1335–1345, https://doi.org/10.1093/jn/113....
31.
Fry R.S., Ashwell M.S., Lloyd K. E., O’Nan A.T., Flowers W.L., Stewart K.R., Spears J.W., 2012. Amount and source of dietary copper affects small intestine morphology, duodenal lipid peroxidation, hepatic oxidative stress,and mRNA expression of hepatic copper regulatory proteins in weanling pigs. J. Anim. Sci. 90, 3112–3119, https://doi.org/10.2527/jas.20....
32.
Gaetke L.M., Chow C.K., 2003. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189, 147–163, https://doi.org/10.1016/S0300-....
33.
Galhardi C.M., Diniz Y.S., Rodrigues H.G., Faine L.A., Burneiko R.C., Ribas B. O., Novelli E. L. B., 2005. Beneficial Effects of dietary copper supplementation on serum lipids and antioxidant defenses in rats. Ann. Nutr. Metab. 49, 283–288, https://doi.org/10.1159/000087....
34.
Gao Y., Yang W., Che D., Adams S., Yang L., 2020. Advances in the mechanism of high copper diets in restraining pigs growth. J. Anim. Physiol. Anim. Nutr. 104, 667–678, https://doi.org/10.1111/jpn.13....
35.
Görlach A., Klappa P., Kietzmann Dr. T., 2006. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 8, 1391–1418, https://doi.org/10.1089/ars.20....
36.
Gromadzka G., Grycan M., Przybyłkowski A.M., 2023. Monitoring of copper in Wilson disease. Diagnostics. 13, 1830, https://doi.org/10.3390/diagno....
37.
Gromadzka G., Tarnacka B., Flaga A., Adamczyk A., 2020. Copper dyshomeostasis in neurodegenerative diseases-therapeutic implications. Int. J. Mol. Sci. 21, 9259, https://doi.org/10.3390/ijms21....
38.
Guo W., Wong S., Xie W., Lei T., Luo Z. 2007. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol.-Endocrinol. Metab. 293, E576–E586, https://doi.org/10.1152/ajpend....
39.
Guo S., Zheng J., Li G., 2022. Effects of growth hormone on lipid metabolism and sexual development in pubertal obese male rats. Open Life Sci. 17, 1531–1540, https://doi.org/10.1515/biol-2....
40.
Gybina A.A., Prohaska J.R., 2008. Copper deficiency results in AMP-activated protein kinase activation and acetylCoA carboxylase phosphorylation in rat cerebellum. Brain Res. 1204, 69–76, https://doi.org/10.1016/j.brai....
41.
Hamann I., Petroll K., Grimm L., Hartwig A., Klotz L.-O., 2014. Insulin-like modulation of Akt/FoxO signaling by copper ions is independent of insulin receptor. Arch. Biochem. Biophys. 558, 42-50, https://doi.org/10.1016/j.abb.....
42.
Hamdi M., Solà D., Franco R., Durosoy S., Roméo A., Pérez J. F., 2018. Including copper sulphate or dicopper oxide in the diet of broiler chickens affects performance and copper content in the liver. Anim. Feed Sci. Technol. 237, 89–97, https://doi.org/10.1016/j.anif....
43.
Hamilton J.P., Koganti L., Muchenditsi A., et al., 2016. Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease in Atp7B−/− (Wilson disease) mice. Hepatology 63, 1828, https://doi.org/10.1002/hep.28....
44.
Hartwig C., Zlatic S. A., Wallin M., Vrailas-Mortimer A., Fahrni C.J., Faundez V., 2019. Trafficking mechanisms of P-type ATPase copper transporters. Curr. Opin. Cell Biol. 59, 24–33, https://doi.org/10.1016/j.ceb.....
45.
He K., Chen Z., Ma Y., Pan Y., 2011. Identification of high-copper-responsive target pathways in Atp7b knockout mouse liver by GSEA on microarray data sets. Mamm. Genome 22, 703–713, https://doi.org/10.1007/s00335....
46.
Helman S.L., Zhou J., Fuqua B.K., Lu Y., Collins J.F., Chen H., Vulpe C.D., Anderson G. J., Frazer D. M., 2023. The biology of mammalian multi-copper ferroxidases. BioMetals 36, 263–281, https://doi.org/10.1007/s10534....
47.
Horn N., Møller L.B., Nurchi V.M., Aaseth J., 2019. Chelating principles in Menkes and Wilson diseases: Choosing the right compounds in the right combinations at the right time. J. Inorg. Biochem. 190, 98–112, https://doi.org/10.1016/j.jino....
48.
Hosseini M.-J., Shaki F., Ghazi-Khansari M., Pourahmad J., 2014. Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem. Biophys. 70, 367–381 https://doi.org/10.1007/s12013....
49.
Hu Z., Liao J., Zhang K., et al., 2023. Effects of long-term exposure to copper on mitochondria-mediated apoptosis in pig liver. Biol. Trace Elem. Res. 201, 1726–1739, https://doi.org/10.1007/s12011....
50.
Huang Y. L., Ashwell M. S., Fry R.S., Lloy K. E., Flowers, W.L., Spears J. W., 2015. Effect of dietary copper amount and source on copper metabolism and oxidative stress of weanling pigs in short-term feeding1. J. Anim. Sci. 93, 2948–2955, https://doi.org/10.2527/jas.20....
51.
Huang C., Chen Q.-L., Luo Z., Shi X., Pan Y.-X., Song Y.-F., Zhuo M.-Q., Wu K., 2014. Time-dependent effects of waterborne copper exposure influencing hepatic lipid deposition and metabolism in javelin goby Synechogobius hasta and their mechanism. Aquat. Toxicol. 155, 291–300, https://doi.org/10.1016/j.aqua....
52.
Huang Y.L., Wang Y., Spears J.W., Lin X., Guo C.H., 2013. Effect of copper on performance, carcass characteristics, and muscle fatty acid composition of meat goat kids. J. Anim. Sci. 91, 5004-5010, https://doi.org/10.2527/jas.20....
53.
Huster D., 2014. Structural and metabolic changes in Atp7b−/− mouse liver and potential for new interventions in Wilson’s disease. Ann. N. Y. Acad. Sci. 1315, 37–44, https://doi.org/10.1111/nyas.1....
54.
Huster D., Finegold M.J., Morgan C.T., Burkhead J.L., Nixon R., Vanderwerf S.M., Gilliam C.T., Lutsenko S., 2006. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am. J. Pathol. 168, 423–434, https://doi.org/10.2353/ajpath....
55.
Huster D., Purnat T.D., Burkhead J.L., Ralle M., Fiehn O., Stuckert F., Olson N.E., Teupser D., Lutsenko S., 2007. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J. Biol. Chem. 282, 8343–8355, https://doi.org/10.1074/jbc.M6....
56.
Ingle A.P., Paralikar P., Shende S., Gupta I., Biswas J.K., da Silva Martins L.H., Rai M., 2018. Copper in medicine: perspectives and toxicity. In: Biomedical Applications of Metals. Springer International, Cham (Swit), pp. 95–112, https://doi.org/10.1007/978-3-....
57.
Kamagate A., Qu, S., Perdomo G., Su D., Kim D.H., Slusher S., Meseck M., Dong H.H., 2008. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118, 2347–2364, https://doi.org/10.1172/JCI329....
58.
Karpenko M.N., Muruzheva Z.M., Ilyechova E.Y., Babich P.S., Puchkova L.V., 2023. Abnormalities in copper status associated with an wlevated risk of Parkinson’s phenotype development. Antioxidants 12, 1654, https://doi.org/10.3390/antiox....
59.
Kaya H., Kaya A., Macit M., Çelebi S., Kaynar Ö., 2018. Effects of dietary copper supplementation on performance, egg quality parameters, yolk cholesterol and fatty acid profiles in laying hens. Indian J. Anim. Res. 52, 1623–1627, https://doi.org/10.18805/ijar.....
60.
Klevay L.M., Hyg S.D., 1973. Hypercholesterolemia in rats produced by an increase in the ratio of zinc to copper ingested. Am. J. Clin. Nutr. 26, 1060–1068, https://doi.org/10.1093/ajcn/2....
61.
Klevay L.M., Inman L., Johnson L.K., Lawler M., Mahalko J.R., Milne D.B., Lukaski H.C., Bolonchuk W., Sandstead H.H., 1984. Increased cholesterol in plasma in a young man during experimental copper depletion. Metabolism 33, 1112–1118, https://doi.org/10.1016/0026-0....
62.
Kim B., Jeong J.Y., Park S.H., Jung H., Kim M., 2022. Effects of dietary copper sources and levels on growth performance, copper digestibility, fecal and serum mineral characteristics in growing pigs. J. Anim. Sci. Technol. 64, 885–896, https://doi.org/10.5187/jast.2....
63.
Krishnamoorthy L., Cotruvo J.A., Chan J., et al., 2016. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 12, 586–592, https://doi.org/10.1038/nchemb....
64.
Lau B.W.C., Klevay L.M., 1981. Plasma lecithin:cholesterol acyltransferase in copper-deficient rats. J. Nutr. 111, 1698–1703, https://doi.org/10.1093/jn/111....
65.
Lawson M.K., Valko M., Cronin M.T.D., Jomová K., 2016. Chelators in iron and copper toxicity. Curr. Pharmacol. Rep. 2, 271–280, https://doi.org/10.1007/s40495....
66.
Lee J.-Y., Kim Y.-H., Kim T.-W., Oh S.-Y., Kim D.-S., Shin B.-S., 2012. New novel mutation of the ATP7B gene in a family with Wilson disease. J. Neurol. Sci. 313, 129–131, https://doi.org/10.1016/j.jns.....
67.
Li Q., Ding X., Kang Y.J., 2014. Copper promotion of angiogenesis in isolated rat aortic ring: role of vascular endothelial growth factor. J. Nutr. Biochem. 25, 44–49, https://doi.org/10.1016/j.jnut....
68.
Li J.S., Li J.L., Wu T.T., 2007. The effects of copper, iron and zinc on digestive enzyme activity in the hybrid tilapia Oreochromis niloticus (L.)× Oreochromis aureus (Steindachner). J. Fish Biol. 71, 1788–1798, https://doi.org/10.1111/j.1095....
69.
Li F., Wu X., Liu H., Zhang B., Liu L., Li F., 2021. Dietary copper supplementation enhances lipolysis in Rex rabbits. J. Trace Elem. Med. Biol. 68, 126851, https://doi.org/10.1016/j.jtem....
70.
Liao P., Shu X., Tang M., Tan B., Yin Y., 2018. Effect of dietary copper source (inorganic vs. chelated) on immune response, mineral status, and fecal mineral excretion in nursery piglets. Food Agric. Immunol. 29, 548–563, https://doi.org/10.1080/095401....
71.
Linder M.C., 1991. Absorption of copper from the digestive tract. In: M.C. Linder (Editor). Biochemistry of Copper. Springer US press. Boston, MA (US), https://doi.org/10.1007/978-1-....
72.
Linder M.C., Hazegh-Azam M., 1996. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 63, 797S–811S, https://doi.org/10.1093/ajcn/6....
73.
Liu Y., Miao J., 2022. An emerging role of defective copper metabolism in heart disease. Nutrients 14, 700, https://doi.org/10.3390/nu1403....
74.
Lutsenko S., Barnes N.L., Bartee M.Y., Dmitriev O.Y., 2007. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 87, 1011–1046, https://doi.org/10.1152/physre....
75.
Lynch S.M., Strain J.J., 1989. Dietary saturated or polyunsaturated fat and copper deficiency in the rat. Biol. Trace Elem. Res. 22, 131–139, https://doi.org/10.1007/BF0291....
76.
Matsumoto M., Han S., Kitamura T., Accili D., 2006. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116, 2464–2472, https://doi.org/10.1172/JCI270....
77.
Mercader J., Iffiú-Soltesz Z., Brenachot X., Földi Á., Dunkel P., Balogh B., Attané C., Valet P., Mátyus P., Carpéné C., 2010. SSAO substrates exhibiting insulin-like effects in adipocytes as a promising treatment option for metabolic disorders. Future Med. Chem. 2, 1735–1749, https://doi.org/10.4155/fmc.10....
78.
Morán-Salvador E., López-Parra M., García-Alonso V., Titos E., Martínez-Clemente M., González-Périz A., López-Vicario C., Barak Y., Arroyo V., Clària J., 2011. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 25, 2538–2550, https://doi.org/10.1096/fj.10-....
79.
NRC, 1994. Nutritional Requirements of Poultry. 9th revised Edition. National Academy Press. Washington, DC (USA).
80.
NRC, 2012. Nutrient Requirements of Swine: 11th Revised Edition. National Academies Press, Washington, DC (USA).
81.
Nguyen H.T.T., Kheravi S.K., Wu S., Roberts J.R., Swick R.A., Toghyani M., 2022. Sources and levels of copper affect liver copper profile, intestinal morphology and cecal microbiota population of broiler chickens fed wheat-soybean meal diets. Sci. Rep. 12, 2249, https://doi.org/10.1038/s41598....
82.
Oe S., Miyagawa K., Honma Y., Harada M., 2016. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell Res. 347, 192–200, https://doi.org/10.1016/j.yexc....
83.
Olivares R. W. I., Postma G.C., Schapira A., Iglesias D.E., Valdez L.B., Breininger E., Gazzaneo P.D., Minatel L., 2019. Biochemical and morphological alterations in hearts of copper-deficient Bovines. Biol. Trace Elem. Res. 189, 447–455, https://doi.org/10.1007/s12011....
84.
Olusi S., Al-Awadhi A., Abiaka C., Abraham M., George S., 2003. Serum copper levels and not zinc are positively associated with serum leptin concentrations in the healthy adult population. Biol. Trace Elem. Res. 91, 137–144, https://doi.org/10.1385/BTER:9....
85.
Onifade A.A., Abu O.A., 1998. Productive response of rabbits to supplemental copper in a diet based on tropical feedstuffs. J. Appl. Anim. Res. 13, 129–135, https://doi.org/10.1080/097121....
86.
Pan Y., Zhu E., Gao X., Nauen R., Xi J., Peng T., Wei X., Zheng C., Shang, Q., 2017. Novel mutations and expression changes of acety-coenzyme A carboxylase are associated with spirotetramat resistance in Aphis gossypii Glover. Insect Mol. Biol. 26, 383–391, https://doi.org/10.1111/imb.12....
87.
Pan Y. X., Zhuo M. Q., Li D. D., Xu Y. H., Wu K., Luo Z., 2019. SREBP-1 and LXRα pathways mediated Cu-induced hepatic lipid metabolism in zebrafish Danio rerio. Chemosphere 215, 370–379, https://doi.org/10.1016/j.chem....
88.
Pesti G.M., Bakalli R.I., 1996. Studies on the feeding of cupric sulfate pentahydrate and cupric citrate to broiler chickens. Poult. Sci. 75, 1086-1091, https://doi.org/10.3382/ps.075....
89.
Polishchuk R., Lutsenko S., 2013. Golgi in copper homeostasis: a view from the membrane trafficking field. Histochem. Cell Biol. 140, 285–295, https://doi.org/10.1007/s00418....
90.
Prohaska J.R., 2008. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 88, 826S–829S, https://doi.org/10.1093/ajcn/8....
91.
Qi Z., Ding S., 2016. Obesity-associated sympathetic overactivity in children and adolescents: the role of catecholamine resistance in lipid metabolism. J. Pediatr. Endocrinol. Metab. 29, 113–125, https://doi.org/10.1515/jpem-2....
92.
Ralle M., Huster D., Vogt S., Schirrmeister W., Burkhead J.L., Capps T.R., Gray L., Lai B., Maryon E., Lutsenko S., 2010. Wilson disease at a single cell level: intracellular copper trafficking acticates compartment- specific responses in hepatocytes *. J. Biol. Chem. 285, 30875–30883, https://doi.org/10.1074/jbc.M1....
93.
Ren F., Logeman B. L., Zhang X., Liu Y., Thiele D. J., Yuan P., 2019. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 10, 1386, https://doi.org/10.1038/s41467....
94.
Rogne M., Taskén K., 2014. Compartmentalization of cAMP signaling in adipogenesis, lipogenesis, and lipolysis. Horm. Metab. Res. 46, 833–840, https://doi.org/10.1055/s-0034....
95.
Sauer V., Siaj R., Todorov T., Zibert A., Schmidt H.H.-J., 2010. Overexpressed ATP7B protects mesenchymal stem cells from toxic copper. Biochem. Biophys. Res. Commun. 395, 307–311, https://doi.org/10.1016/j.bbrc....
96.
Seessle J., Gohdes A., Gotthardt D.N., Pfeiffenberger,J., Eckert N., Stremmel W., Reuner U., WeissK. H., 2011. Alterations of lipid metabolism in Wilson disease. Lipids Health Dis. 10, 83, https://doi.org/10.1186/1476-5....
97.
Skrivan M., Skrivanova V., Marounek M., Tumova E., Wolf J., 2000. Influence of dietary fat source and copper supplementation on broiler performance, fatty acid profile of meat and depot fat, and on cholesterol content in meat. Br. Poult. Sci. 41, 608-614, https://doi.org/10.1080/713654....
98.
Smith S., 1994. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. The FASEB J. 8, 1248-1259, https://doi.org/10.1096/fasebj....
99.
Solaiman S.G., Shoemaker C.E., Jones W.R., Kerth C.R., 2006. The effects of high levels of supplemental copper on the serum lipid profile, carcass traits, and carcass composition of goat kids. J. Anim. Sci. 84, 171–177, https://doi.org/10.2527/2006.8....
100.
Song Y.-F., Huang C., Shi X., Pan Y.-X., Liu X.,Luo Z., 2016a. Endoplasmic reticulum stress and dysregulation of calcium homeostasis mediate Cu-induced alteration in hepatic lipid metabolism of javelin goby Synechogobius hasta. Aquat. Toxicol. 175, 20–29, https://doi.org/10.1016/j.aqua....
101.
Song Y.-F., Luo Z., Zhang L.-H., Hogstrand C., Pan Y.-X., 2016b. Endoplasmic reticulum stress and disturbed calcium homeostasis are involved in copper-induced alteration in hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Chemosphere 144, 2443–2453, https://doi.org/10.1016/j.chem....
102.
Song M., Schuschke D.A., Zhou Z., Chen T., Pierce W.M., Wang R., Johnson W.T., McClain C.J., 2012. High fructose feeding induces copper deficiency in Sprague-Dawley rats: A novel mechanism for obesity related fatty liver. J. Hepatol. 56, 433–440, https://doi.org/10.1016/j.jhep....
103.
Su R., Wang R., Cao H., Pan J., Chen L., Li C., Shi D., Tang Z., 2011. High copper levels promotes broiler hepatocyte mitochondrial permeability transition in vivo and in vitro. Biol. Trace Elem. Res. 144, 636–646, https://doi.org/10.1007/s12011....
104.
Svensson P.-A., Englund M.C.O., Markström E., Ohlsson B.G., Jernås M., Billig H., Torgerson J.S., Wiklund O., Carlsson L.M.S., Carlsson B., 2003. Copper induces the expression of cholesterogenic genes in human macrophages. Atherosclerosis 169, 71–76, https://doi.org/10.1016/S0021-....
105.
Tailleux A., Wouters K., Staels B., 2012. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1821, 809–818, https://doi.org/10.1016/j.bbal....
106.
Tang Z., Gasperkova D., Xu J., Baillie R., Lee J.-H., Clarke S.D., 2000. Copper deficiency induces hepatic fatty acid synthase gene transcription in rats by increasing the nuclear content of mature sterol regulatory element binding protein 1. J. Nutr. 130, 2915–2921, https://doi.org/10.1093/jn/130....
107.
Tosco A., Fontanella B., Danise R., Cicatiello L., Grober O.M.V., Ravo M., Weisz A., Marzullo L., 2010. Molecular bases of copper and iron deficiency-associated dyslipidemia: a microarray analysis of the rat intestinal transcriptome. Genes Nutr. 5, 1–8, https://doi.org/10.1007/s12263....
108.
Tümer Z., Møller L. B., 2010. Menkes disease. Eur. J. Hum. Genet. 18, 511–518, https://doi.org/10.1038/ejhg.2....
109.
Villagómez-Estrada S., Pérez J.F., Darwich L., Vidal A., van Kuijk S., Melo-Durán D., Solà-Oriol D., 2020. Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J. Anim. Sci. 98, skaa117, https://doi.org/10.1093/jas/sk....
110.
Walter P.L., Kampkötter A., Eckers A., Barthel A., Schmoll D., Sies H., Klotz L.-O., 2006. Modulation of FoxO signaling in human hepatoma cells by exposure to copper or zinc ions. Arch. Biochem. Biophys. 454, 107–113, https://doi.org/10.1016/j.abb.....
111.
Wang B., Feng L., Jiang W.-D., et al., 2015. Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-κB, TOR and Nrf2 signaling molecules in the gills of fish: Preventive role of arginine. Aquat. Toxicol. 158, 125–137, https://doi.org/10.1016/j.aqua....
112.
Wang H., Zhang R., Shen J., Jin Y., Chang C., Hong M., Guo S., He D., 2023. Circulating level of blood iron and copper associated with inflammation and disease activity of rheumatoid arthritis. Biol. Trace Elem. Res. 201, 90–97, https://doi.org/10.1007/s12011....
113.
Wang Y., Zhang W., Yao Q., 2021. Copper-based biomaterials for bone and cartilage tissue engineering. J. Orthop. Transl. 29, 60–71, https://doi.org/10.1016/j.jot.....
114.
Wang K., Zhou W., Hu G., Wang L., Cai R., Tian T., 2023. TFEB SUMOylation in macrophages accelerates atherosclerosis by promoting the formation of foam cells through inhibiting lysosomal activity. Cell. Mol. Life Sci. 80, 358, https://doi.org/10.1007/s00018....
115.
Wang H., Zhu H., Wang X., Li E., Du Z., Qin J., Chen L., 2018. Comparison of copper bioavailability in copper-methionine, nano-copper oxide and copper sulfate additives in the diet of Russian sturgeon Acipenser gueldenstaedtii. Aquaculture. 482, 146–154, https://doi.org/10.1016/j.aqua....
116.
Wang J., Zhu X., Guo Y., Wang Z., Zhao B., Yin Y., Liu, G., 2016. Influence of dietary copper on serum growth-related hormone levels and growth performance of weanling pigs. Biol. Trace Elem. Res. 172, 134–139, https://doi.org/10.1007/s12011....
117.
Wei Y., Wang D., Topczewski F., Pagliassotti M.J., 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol.-Endocrinol. Metab. 291, E275–E281, https://doi.org/10.1152/ajpend....
118.
Wen A., Dai S., Wu X., Cai Z., 2019. Copper bioavailability, mineral utilization, and lipid metabolism in broilers. Czech J. Anim. Sci. 64, 483–490, https://doi.org/10.17221/210/2....
119.
Wen Y., Li R., Piao X., Lin G., He P., 2022. Different copper sources and levels affect growth performance, copper content, carcass characteristics, intestinal microorganism and metabolism of finishing pigs. Anim. Nutr. 8, 321–330, https://doi.org/10.1016/j.anin....
120.
Werstuck G.H., Lentz S.R., Dayal S., et al., 2001. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest. 107, 1263–1273, https://doi.org/10.1172/JCI115....
121.
Wijmenga C., Klomp L.W., 2004. Molecular regulation of copper excretion in the liver. Proceedings of the Nutrition Society. 63, 31–39, https://doi.org/10.1079/PNS200....
122.
Wright L.M., Huster D., Lutsenko S., Wrba F., Ferenci P., Fimmel C.J., 2009. Hepatocyte GP73 expression in Wilson disease. J. Hepatol. 51, 557–564, https://doi.org/10.1016/j.jhep....
123.
Wu X., Dai S., Hu J., Hu H., Wang S., Wen A., 2019. Influence of dietary copper methionine concentrations on growth performance, digestibility of nutrients, serum lipid profiles, and immune defenses in broilers. Biol. Trace Elem. Res. 191, 199–206, https://doi.org/10.1007/s12011....
124.
Wu X., Zhu M., Jiang Q., Wang L., 2020. Effects of copper sources and levels on lipid profiles, immune parameters, antioxidant defenses, and trace element residues in broilers. Biol. Trace Elem. Res. 194, 251–258, https://doi.org/10.1007/s12011....
125.
Xue X., Piao J.-H., Nakajima A., Sakon-Komazawa S., Kojima Y., Mori K., Yagita H., Okumura K., Harding H., Nakano H., 2005. Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J. Biol. Chem. 280, 33917–33925, https://doi.org/10.1074/jbc.M5....
126.
Yang F., Liao J., Yu W., Pei R., Qiao N., Han Q., Hu L., Li Y., Guo J., Pan J., Tang Z., 2020. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol. Environ. Saf. 200, 110715, https://doi.org/10.1016/j.ecoe....
127.
Yang H., Ralle M., Wolfgang M.J., Dhawa N., Burkhead J.L., Rodriguez S., Kaplan J.H., Wong G.W., Haughey N., Lutsenko S., 2018. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLOS Biol. 16, e2006519, https://doi.org/10.1371/journa....
128.
Yang W., Wang J., Liu L., Zhu X., Wang X., Liu Z., Wang Z., Yang L., Liu, G., 2011. Effect of high dietary copper on somatostatin and growth hormone-releasing hormone levels in the hypothalami of growing pigs. Biol. Trace Elem. Res. 143, 893–900, https://doi.org/10.1007/s12011....
129.
Yang W., Zhao C., Zhang C., Yang L., 2016. High dietary copper increases catecholamine concentrations in the hypothalami and midbrains of growing pigs. Biol. Trace Elem. Res. 170, 115–118, https://doi.org/10.1007/s12011....
130.
Yurkova I.L., Arnhold J., Fitzl G., Huster D., 2011. Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b−/− mouse model of Wilson’s disease. Chem. Phys. Lipids 164, 393–400, https://doi.org/10.1016/j.chem....
131.
Zangiabadi S., Chamoun K.P., Nguyen K., Tang Y., Sweeney G., Abdul-Sater A.A., 2023. Copper infused fabric attenuates inflammation in macrophages. Plos one. 18, e0287741, https://doi.org/10.1371/journa....
132.
Zelcer N., Tontonoz P., 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614, https://doi.org/10.1172/JCI278....
133.
Zhang Y., Dong Z., Yang H., Liang X., Zhang S., Li X., Wan D., Yin Y., 2020. Effects of dose and duration of dietary copper administration on hepatic lipid peroxidation and ultrastructure alteration in piglets’ model. J. Trace Elem. Med. Biol. 61, 126561, https://doi.org/10.1016/j.jtem....
134.
Zhong G., Li Y., Ma F., Huo Y., Liao J., Han Q., Hu L., Tang Z., 2023. Copper exposure induced chicken hepatotoxicity: involvement of ferroptosis mediated by lipid peroxidation, ferritinophagy, and inhibition of FSP1-CoQ10 and Nrf2/SLC7A11/GPX4 axis. Biol. Trace Elem. Res. 202, 1711-1712, https://doi.org/10.1007/s12011....
135.
Zhong C.-C., Zhao T., Hogstrand C., Chen F., Song C.-C., Luo Z., 2022. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J. Nutr. Biochem. 100, 108883, https://doi.org/10.1016/j.jnut....
136.
Zhu Q.-L., Luo Z., Zhuo M.-Q., Tan X.-Y., Sun L.-D., Zheng J.-L., Chen Q.-L., 2014. In vitro exposure to copper influences lipid metabolism in hepatocytes from grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 40, 595-605, https://doi.org/10.1007/s10695....
137.
Zischka H., Lichtmannegger J., Schmit S., Jägemann N., et al., 2011. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J. Clin. Invest. 121, 1508-1518, https://doi.org/10.1172/JCI454....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.